Abstract
Small cell carcinoma in the urinary bladder is very rare. There are only a handful of cases reported in the medical literature to date. A case of a primary bladder small cell carcinoma, detected by magnetic resonance imaging (MRI) during a follow up examination of a 77-year-old male patient who visited the outpatient clinic of our institution initially complaining of voiding difficulties and diagnosed with adenocarcinoma of the prostate gland with a Gleason score of 10 is, herein, reported.
Small cell carcinoma in the urinary bladder is indeed very rare and unusual disease. Only handful of cases has been reported in respectful literatures.
A male with voiding difficulties that had been progressed came into outpatient clinic of our institution in February 2006. Past history and family review revealed no remarkable findings. Physical examination including digital rectal examination (DRE) discovered enlarged prostate gland with the size of more than approximately 40 g. International Prostate Symptom Score (IPSS) of 19 and the level of prostate-specific antigen (PSA) of 21.73 were discovered and followed by transrectal ultrasonography (TRUS) of the prostate gland that revealed the volume of 61 cc, that is, prostate-specific antigen density of 0.36, with a noticeable hypoechoic lesion in the peripheral zone. TRUS-guided needle biopsy of the prostate gland was, thus, carried out exhibiting adenocacinoma with Gleason score of 10 (5+5), ranging from the score of 7 at left apex to the score of 10 at right base, mid and apex (Fig. 1). Results of cytology including cytospin continuously reported clusters of atypical cells, consistent with carcinoma. Imaging modalities of prostate magnetic resonance imaging (MRI) (Fig. 2) and radionuclide bone scan was conducted to evaluate the stage of prostate cancer. T2 weighted image of prostate MRI revealed a lesion with diffuse low signal intensity at both peripheral zones from apex to base and both seminal vesicle as well as a lesion suspicious of invasion into the bladder neck without definite image of any mass in the bladder in addition to several enlarged obturator and right external iliac lymph nodes. Any involvement of upper urinary tract was not evidenced by radionuclide bone scan that unveiled traumatic rib lesions.
The patient was diagnosed as having stage T4aN2M0 of prostate cancer and was immediately begun with hormonal therapy of bicalutamide and leuproreline acetate. In the course of treatment, the patient was readmitted in June 2006 with painless gross hematuria and worsened voiding difficulties that was alleviated with alpha-blocker developed in May 2006. Physical examination discovered multiple palpable masses located in suprapubic area with increased serum creatine level in laboratory result. Kidney and bladder ultrasonography was, then, performed unveiling both hydronephrosis. Failure of indwelling ureteral catheter on both sides, due to multiple bullous masses and severe degree of erythematous trabeculation in the bladder hampering the procedure of locating ureteral orfices, ensure the procedure of percutaneous nephrostomy on both sides. Abdominopelvic computed tomography (CT) disclosed about 14.5×10.3 cm sized multilobulated heterogenous enhancing soft tissue mass in pelvic cavity, involving bladder and prostate gland that was not visible in previous study of prostate MRI, as well as a hepatic mass suspicious of metastasis at both lobes. Enlarged lymph nodes were also detected in obturator area and perilesional area with increased size of previously detected right external iliac lymph node.
Cystoscopy was performed owing to persistent hematuria and specimen for biopsy was obtained from multiple bullous masses in the bladder. According to the report of biopsy, microscopically, cells were consistent with finding of small cell carcinoma without evidence of transitional cell or squamous cell carcinoma. Study of immunohistochemistry (Fig. 3) was conducted to confirm neuroendocrine activity of small cell carcinoma and showed the tumor cells were positive for CD56, weakly positive for synaptophysin, while negative for cytokeratin, chromogranin, leukocyte common antigen (LCA), T-cell (UCHL-1), CD3, CD20, and PSA.
Thoracic CT was available to detect any other primary and metastatic lesion in lung and other organs and visualized calcified nodule in left upper lobe with fibrotic bands, probably, sequelae of tuberculosis, a tiny dense nodule in right upper lobe, suspicious of granuloma, focal bronchiectasis with linear atelectasis in right middle lobe and small sized lymph nodes in mediastinum. The patient refused further treaments and was lost during follow up period while additional treatment options were considered.
Primary small cell carcinoma is known to occur mainly in the urinary bladder and the prostate gland, in urologic field, with incidence of approximately 0.5% to 1% and mean age of 66 years.1,2 Small cell carcinoma of the prostate gland, once occurred, has been known to have a worse prognosis than that of the bladder.2 In Korea, to this date, three cases of small cell carcinoma in the urinary bladder including a 66-year-old male have been reported.3 There has been no reported case of adenocarcinoma of the gland concomitantly occurred with small cell carcinoma of the bladder. However, small cell carcinoma of the gland could be detected if repeat biopsy or further additional study of the gland were conducted since adenocarconima was discovered in initially conducted sextant needle biopsy. Primary small cell carcinoma of the bladder behaves as if other small cell carcinoma in other organs that it progresses quickly and has metastatic lesions when it is diagnosed. Radical surgery combined with chemotherapy has been accepted as mainstream of treatment options of surgery, chemotherapy or radiation therapy.4,5
Abrahams et al1 noted various information of sex, location of mass in the bladder and the presence of concomitant urothelial carcinoma, after retrospective study of 51 patients with small cell carcinoma in the bladder. According to Choong et al,5 radical surgery is beneficial to patients with TNM stage II and combination therapy of radical surgery and chemotherapy or chemotherapy alone is advantageous to patients with TNM stage III and IV, with regard to improved survival rate, after reviewing total of 44 cases of primary small cell carcinoma of the bladder. However, according to Abrahams et al1, clinical stage of small cell carcinoma of the bladder alone could not effectively affect the survival rate, instead, micrometastasis could an influencing factor of mortality of the patient. Podesta and True6 reported that there has been no difference in prognosis of the patient between radical cystectomy and partial cystectomy.
Chemotherapy is mainly based on combination regimen of platinum and cisplatin, adjunct with other regimens such as methotrexate, vinblastine, doxorubicin and etoposide, cited by Mangar et al,7 Siefker-Radtke et al8 also reported usefulness of chemotherapy prior to planning of any surgery as a treatment of small cell carcinoma. Primary small cell carcinoma of the bladder is a rare fatal disease with quick progression and metastasis. When considering at above, combination chemotherapy after radical surgery is the best choice for improving the survival rate of the patients undergoing small cell carcinoma of the bladder by currently. Gemcitabine and Cisplatin combination chemotherapy regimen that was proven as an effective treatment for the bladder and ureter carcinoma can also be considered as those for the small cell carcinoma of the bladder.
Figures and Tables
Fig. 1
An image of the transrectal ultrasonography (TRUS) of the prostate gland and result of the TRUS-guided needle biopsy of the prostate gland. (A) A hypoechoic lesion in the peripheral zone is noted (arrow). (B) Adenocarcinoma of the prostate gland (Hematoxylin-Eosin (HE) stain, ×100).

Fig. 2
Images of the prostate gland by magnetic resonance imaging (MRI). Diffuse low signal intensity at both peripheral zones from apex to base and both seminal vesicles in the T2 weighted image.
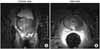
Fig. 3
Immunohistochemical staining confirmed the neuroendocrine activity of the small cell carcinoma and showed that the tumor cells were positive for CD56, weakly positive for synaptophysin, while negative for cytokeratin, chromogranin, leukocyte common antigen (LCA), T-cell (UCHL-1), CD3, CD20, and prostate-specific antigen (PSA) (HE stain, ×400).

References
1. Abrahams NA, Moran C, Reyes AO, Siefker-Radtke A, Ayala AG. Small cell carcinoma of the bladder: a contemporary clinopathological study of 51 cases. Histopathology. 2005. 46:57–63.
2. Mackey JR, Au HJ, Hugh J, Venner P. Genitourinary small cell carcinoma: determination of clinical and therapeutic factors associated with survival. J Urol. 1998. 159:1624–1629.
3. Sung DJ, Lee JG, Cho JH. A case of small cell carcinoma of the urinary bladder. Korean J Urol. 1994. 35:1137–1141.
4. Sved P, Gomez P, Manoharan M, Civantos F, Soloway MS. Small cell carcinoma of the bladder. BJU Int. 2004. 94:12–17.
5. Choong NW, Quevedo JF, Kaur JS. Small cell carcinoma of the urinary bladder. The Mayo Clinic experience. Cancer. 2005. 103:1172–1178.
6. Podesta AH, True LD. Small cell carcinoma of the bladder. Report of five cases with immunohistochemistry and review of the literature with evaluation of prognosis according to stage. Cancer. 1989. 64:710–714.
7. Mangar SA, Logue JP, Shanks JH, Cooper RA, Cowan RA, Wylie JP. Small-cell carcinoma of the urinary bladder: 10-year experience. Clin Oncol (R Coll Radiol). 2004. 16:523–527.
8. Siefker-Radtke AO, Dinney CP, Abrahams NA, Moran C, Shen Y, Pisters LL, et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M.D. Anderson cancer experience. J Urol. 2004. 172:481–484.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download