Abstract
Purpose
The aim of this study was to investigate the efficacy and tolerability of extended-release oxybutynin (oxybutynin ER) in children with a neurogenic bladder.
Materials and Methods
Fifty-four patients (21 myelomeningocele and 33 lipomyelomeningocele) with a neurogenic bladder were enrolled in the study. We reviewed the medical records and performed a telephone interview. The treatments were changed from immediate-release oxybutynin (oxybutynin IR) or other anticholinergics to oxybutynin ER from August to December 2006. The mean age of the study patients was 11.1 years (range 4 to 18 years) and the mean body weight was 37.9kg (range 16.2 to 72.0kg). All patients were asked about the effectiveness, side effects and compliance with the medication. The number of voids, volume of urine per void or clean intermittent catheterization (CIC) and number of incontinence episodes were also evaluated.
Results
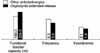
The mean duration of oxybutynin ER treatment was 16.3 weeks (range 7-25 weeks). Twenty-six patients (48.1%) responded they had improvement in voiding symptoms. Among the patients, there was a significant reduction in the number of incontinence episodes (from 3.3 to 1.3, p<0.001) with the change in medications. The number of voids or CIC per 24 hours and the maximum volume of urine per void or CIC did not show a significant change. Another twenty-eight patients (51.9%) responded that the improvements were maintained. Among these patients, there were no significant changes of the medications. Only five patients (9.3%) changed their medication because of the side effects.
Figures and Tables
 | Fig. 1The efficacy of extended-release oxybutynin. In this group, the patients said they experienced improvement in efficacy after the medication was changed from other anticholinergics to the extended-release oxybutynin (p>0.05 except incontinence). |
 | Fig. 2The efficacy of extended-release oxybutynin. In this group, the patients said they experienced maintenance of the improved efficacy after the medication change from other anticholinergics to the extended-release oxybutynin (p>0.05). |
References
1. Yoshimura N, Chancellor MB. Current and future pharmacological treatment for overactive bladder. J Urol. 2002. 168:1897–1913.
2. Baskin LS, Kogan BA, Benard F. Treatment of infants with neurogenic bladder dysfunction using anticholinergic drugs and intermittent catheterisation. Br J Urol. 1990. 66:532–534.
3. Kasabian NG, Bauer SB, Dyro FM, Colodny AH, Mandell J, Retik AB. The prophylactic value of clean intermittent catheterization and anticholinergic medication in newborns and infants with myelodysplasia at risk of developing urinary tract deterioration. Am J Dis Child. 1992. 146:840–843.
4. Park JM, Bauer SB, Freeman MR, Peters CA. Oxybutynin chloride inhibits proliferation and suppresses gene expression in bladder smooth muscle cells. J Urol. 1999. 162:1110–1114.
5. Appel RA. Oxybutynin. The clinical experience. Contemp Urol. 1991. 3:60–67.
6. Yarker YE, Goa KL, Fitton A. Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties and its therapeutic use in detrusor instability. Drugs Aging. 1995. 6:243–262.
7. Massad CA, Kogan BA, Trigo-Rocha FE. The pharmacokinetics of intravesical and oral oxybutynin chloride. J Urol. 1992. 148:595–597.
8. Kasabian NG, Vlachiotis JD, Lais A, Klumpp B, Kelly MD, Siroky MB, et al. The use of intravesical oxybutynin chloride in patients with detrusor hypertonicity and detrusor hyperreflexia. J Urol. 1994. 151:944–945.
9. Palmer LS, Zebold K, Firlit CF, Kaplan WE. Complications of intravesical oxybutynin chloride therapy in the pediatric myelomeningocele population. J Urol. 1997. 157:638–640.
10. Siddiqui MA, Perry CM, Scott LJ. Oxybutynin extended-release: a review of its use in the management of overactive bladder. Drugs. 2004. 64:885–912.
11. Anderson R, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS. OROS Oxybutynin Study Group. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. J Urol. 1999. 161:1809–1812.
12. Versi E, Appell R, Mobley D, Patton W, Saltzstein D. The Ditropan XL Study Group. Dry mouth with conventional and controlled-release oxybutynin in urinary incontinence. Obstet Gynecol. 2000. 95:718–721.
13. Gleason DM, Susset J, White C, Munoz DR, Sand PK. Ditropan XL Study Group. Evaluation of a new once-daily formulation of oxybutynin for the treatment of urinary urge incontinence. Urology. 1999. 54:420–423.
14. Youdim K, Kogan BA. Preliminary study of the safety and efficacy of extended-release oxybutynin in children. Urology. 2002. 59:428–432.
15. Reinberg Y, Crocker J, Wolpert J, Vandersteen D. Therapeutic efficacy of extended release oxybutynin chloride, and immediate release and long acting tolterodine tartrate in children with diurnal urinary incontinence. J Urol. 2003. 169:317–319.
16. Kaefer M, Pabby A, Kelly M, Darbey M, Bauer SB. Improved bladder function after prophylactic treatment of the high risk neurogenic bladder in newborns with myelomeningocele. J Urol. 1999. 162:1068–1071.
17. Thompson IM, Lauvetz R. Oxybutynin in bladder, spasm, neurogenic bladder and enuresis. Urology. 1976. 8:452–454.
18. Nijman RJ. Classification and treatment of functional incontinence in children. BJU Int. 2000. 85:Suppl 3. 37–42.
19. Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004. 3:46–53.
20. Douchamps J, Derenne F, Stockis A, Gangji D, Juvent M, Herchuelz A. The pharmacokinetics of oxybutynin in man. Eur J Clin Pharmacol. 1988. 35:515–520.
21. Gupta S, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate release oxybutynin. J Clin Pharmacol. 1999. 39:289–296.
22. Autret E, Jonville AP, Dutertre JP, Bertiere MC, Robert M, Averous M, et al. Plasma levels of oxybutynin chloride in children. Eur J Clin Pharmacol. 1994. 46:83–85.
23. Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990. 150:1881–1884.
24. Paes AH, Bakker A, Soe-Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care. 1997. 20:1512–1517.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download