Abstract
Purpose
Even though hypospadias is one of the most common congenital anomalies, the cause of hypospadias is largely unknown. With regard to molecular biology and microarray technology, it appears that hypospadias is potentially related to disrupted gene expression. Genomic analysis of hypospadiac tissue indicated a potential role for activating transcription factor 3 (ATF3) in the development of this anomaly. This study prospectively examined the expression of ATF3 in tissues from 20 children with hypospadias compared with 26 normal penile skin tissue samples from elective circumcision.
Materials and Methods
Prepucial tissue was obtained from children who underwent repair of hypospadias for comparison with tissue samples from children who underwent elective circumcision. Skin specimens were evaluated for the expression of ATF3 protein by immunohistochemical staining.
Hypospadias is defined as an ectopic urethral meatus on the ventral aspect of the penis, scrotum, or perineum with or without ventral penile curvature, and estimates of its prevalance range from 3 to 8 cases per 1,000 male births [1-3]. Its etiology is incompletely understood. Hypospadias probably has a multifactorial origin that involves the actions of environmental factors and endocrine-active compounds against a genetic backdrop, although few molecular and mechanistic studies have been done [4,5].
Activating transcription factor 3 (ATF3) is a member of the ATF/cAMP responsive element binding (CREB) family [6]. ATF3 may be involved in homeostasis, wound healing, cell adhesion, cancer cell invasion, apoptosis, and signaling pathways [7,8]. Overexpression of ATF3 protein suppresses cell growth and slows the transition of cells from G1 to S phase. This evidence suggests that the ATF3 protein may play a negative role in cell cycle progression [9,10]. Microarray results indicate that ATF3 is one of the genes that is upregulated in hypospadiac tissue [11,12].
In this study, the role of expression of ATF3 protein was investigated in the etiology of hypospadias.
Following institutional review board approval, informed consent was received from parents. Twenty-five males aged between 5 and 9 years (mean: 7.4 years) with hypospadias who were scheduled to undergo surgical repair were prospectively entered into the study from December 2008 to June 2009. Patients with an undescended testis, intersex condition, or known endocrine abnormalities; who had undergone testosterone replacement therapy preoperatively; or who had a family history of hypospadias or a maternal age higher than 35 at the time of birth were excluded from the study. The position of the urethral meatus was assessed according to the Ducket clasification by a single surgeon [13]. Excess prepucial tissue was obtained from ethnically comparable boys at the time of elective surgery for hypospadias. Controls consisted of 26 patients aged between 6 and 11 years (mean: 8.3 years) undergoing elective circumsicion. Tissue specimens were prepared for parafin-embedded or frozen sectioning. We included only cases from Turkey and controls living in the same area.
Skin specimens were evaluated for the expression of ATF3 protein by immunohistochemical staining by use of the standard protocol accompanying the ABC kit (Vector Laboratories, Inc., CA, USA) with overnight primary-antibody incubation with rabbit-anti-ATF3 (Santa Cruz Biotechnology, Inc., CA, USA) at a dilution of 1:400. Control sections were incubated without primary antibody. Specimens were examined under light microscopy with hemotoxylen dye by two independent pathologists blinded to the specimens.
Nuclear ATF3 immunoreactivity was considered abnormal when samples demonstrated more than 20% nuclear reactivity. If the immunoreactivity of the specimen was greater than 50%, it was recorded as strong intensity by the agreement of two observers.
Fisher's exact two-tailed tests were used to compare the frequency data of the two groups. Statistical significance was assigned at p<0.05. To evaluate inter- and intraobserver agreement on immunohistochemical analysis, Cohen kappa statistical analysis was performed by using SPSS for Windows version 15.0 (SPSS Inc., Chicago, IL, USA).
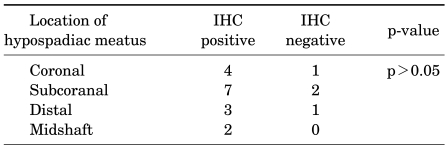
Forty-six patients were enrolled in this study. Five of the 25 hypospadiac patients were excluded because of a history of previous orchidopexy, testosterone replacement therapy, or diagnosed concomitant undescended testis. The location of the meatus in the hypospadiac patients is presented in Table 1. None of the hypospadiac patients had a proximally located meatus (Table 1).
ATF3 protein was localized in the nuclei of stromal cells and the vascular endothelium in the subcutaneous tissue of the prepuce. All of the patients had dorsal prepitual skin. Of the 46 specimens examined by immunohistochemistry, 16 (80%) of the 20 patients with hypospadias showed expression of ATF3 protein, and only 3 (11%) of the 26 patients undergoing circumcision stained positive (p<0.05) (Table 2). Intra- and interobserver agreement was excellent with kappa (Cohen statistics) values of 0.88 and 0.80, respectively.
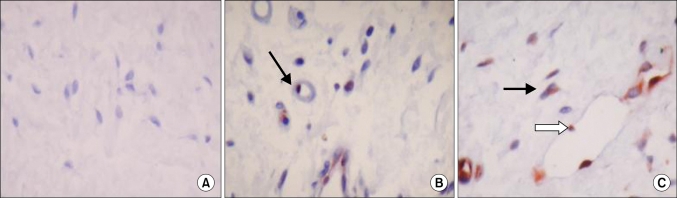
The immunoreactivity of expression of ATF3 protein in normal tissue and in a hypospadiac sample are shown in Fig. 1. The staining intensities were 6.9 times as strong in patients in the hypospadiac group as in the control group (RR: 6.933, 95% CI: 2.3-11.2). The staining intensities were found to be similiar in patients with distal and middle hypospadias (p>0.05) (Table 3). Strong staining intensities were found in 2 patients with a meatus located midshaft, whereas strong staining intensities were found in 2 patients with a distally located urethral meatus.
The development of the male external genitalia is a complex process, comprising genetic programming, cell differentiation, hormonal signaling, enzyme activity, and tissue remodeling, which follows an orderly sequence, occurring in a time- and concentration-dependent manner [14]. Any disturbance in these processes can lead to hypospadias. The cause of hypospadias is largely unknown, however. Several etiologies for hypospadias have been suggested, including genetic, endocrine, and environmental factors [4,5].
There are conflicting results in the literature about the association between ATF3 and hypospadias. The first human study to demonstrate a relationship between ATF3 and hypospadias was performed by Liu et al [15]. Immunohistochemical analysis of human foreskin demonstrated that 86% of the hypospadias samples were positive for the expression of ATF3, whereas only 13% of those from normal tissues were positive in their study. Beleza-Meireles et al analyzed DNA from 330 boys with nonsyndromic hypospadias [16]. ATF3 as a hormone-responsive gene has been shown to be related to the etiology of hypospadias. However, it is a well-known fact that most associations reported in genetic studies do not replicate across subsequent studies [17]. van der Zanden et al were unable to replicate the results of earlier studies [18]. Analyzed populations in mentioned studies were indeed dissimilar, and some of the populations even differed in ethnicity. We included only a Turkish population in this study.
This study demonstrated that ATF3 protein was overexpressed in an ethnically similiar hypospadiac pediatric age group. Conventional manual immunohistochemical methods were used because of cost, time, and space considerations to detect abnormal immunoreactivity. The cutoff point was chosen as 20% for abnormal immunoreactivity because sequential testing of different cutoffs from 0% to 95% in 5% increments revealed that 20% was the best cutoff for abnormal protein expression. The staining intensities appeared to be stronger in patients with more severe hypospadias than in those with mild hypospadias. All patients with a midshaft-located urethral meatus showed stronger intensities, whereas 3 of 18 distal hypospadiac patients showed strong intensities of ATF3 protein expression.
Most of the hypospadiac patients in this study were recorded as having a mild degree of hypospadias, and there were no patients with a proximally located meatus. The staining intensities were found to be stronger in patients in the hypospadiac group than in the control group. ATF3 overexpression may have been related to the severity of hypospadias in our study. None of the hypospadiac patients with a coronal or subcoranal meatus showed strong staining intensities. However, all of the hypospadiac patients with a midshaft-located meatus showed strong staining intensities.
There may be a difference in the etiology of hypospadias in different populations. A growing body of evidence suggests that the development of hypospadias has a two-hit etiology involving a genetic predisposition coupled with fetal exposure to an environmental disruptor [14]. Endocrine-related genes, namely androgen receptor gene (AR), encoding estrogen receptor 1 (ESR1) and ATF3, have been associated with hypospadias in previous studies [16,19,20]. However, the numbers of samples analyzed in these studies were relatively small. In a large study population, four single-nucleotide polymorphism genes including ATF3 were investigated by van der Zanden et al [18]. They were unable to show an association between hyospadias and ATF3. Differences in results between their and previous studies may also have been caused by disease heterogeneity due to differences in the criteria used to select the cases. In this study, we strictly excluded factors suspected to be of relevance in the etiology of hypospadias. Population stratification, which occurs when cases and controls are drawn from different subgroups that differ in disease prevalence and frequency of the genetic variant, may lead to spurious results. We selected only cases and controls living in the same area in Turkey to minimize the chance of population stratification.
The current study is not devoid of limitations. The power of our conclusion may be somewhat limited by the small study population. The clinical application of immunohistochemistry has been limited by discrepancies related to variability in the interpretation and stratification criteria and inconsistency in specimen handling and technical procedures. However, two blinded pathologists graded the blocks to minimize interpreter-related variability in this study. An automated immunostaining (ACIS) platform and quantitative imaging may also help to minimize technique-related variability, but the major drawback to this technology is the apparent lack of an agreed-on or clearly described standard ACIS scoring method.
ACKNOWLEDGEMENT
We are greateful to Dr. Hamit Okur for providing excellent collaboration between our Department and the Department of Pediatric Surgery.
Notes
References
1. Borer JG, Retik AB. Wein AJ, Kavuoussi LR, Novick AC, Partin AW, Peters CA, editors. Hypospadias. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;p. 2284–2327.
2. Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics. 1997; 100:831–834. PMID: 9346983.

3. Carmichael SL, Shaw GM, Nelson V, Selvin S, Torfs CP, Curry CJ. Hypospadias in California: trends and descriptive epidemiology. Epidemiology. 2003; 14:701–706. PMID: 14569186.
4. Steinhardt GF. Endocrine disruption and hypospadias. Adv Exp Med Biol. 2004; 545:203–215. PMID: 15086029.

5. Willingham E, Baskin LS. Candidate genes and their response to environmental agents in the etiology of hypospadias. Nat Clin Pract Urol. 2007; 4:270–279. PMID: 17483812.

7. Yin X, Dewille JW, Hai T. A potential dichotomous role of ATF3, an adaptive-response gene, in cancer development. Oncogene. 2008; 27:2118–2127. PMID: 17952119.

8. Thompson MR, Xu D, Williams BR. ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med. 2009; 87:1053–1060. PMID: 19705082.

9. Kawauchi J, Zhang C, Nobori K, Hashimoto Y, Adachi MT, Noda A, et al. Transcriptional repressor activating transcription factor 3 protects human umbilical vein endothelial cells from tumor necrosis factor-alpha-induced apoptosis through down-regulation of p53 transcription. J Biol Chem. 2002; 277:39025–39034. PMID: 12161427.
10. Zhang C, Gao C, Kawauchi J, Hashimoto Y, Tsuchida N, Kitajima S. Transcriptional activation of the human stress-inducible transcriptional repressor ATF3 gene promoter by p53. Biochem Biophys Res Commun. 2002; 297:1302–1310. PMID: 12372430.

11. Nobori K, Ito H, Tamamori-Adachi M, Adachi S, Ono Y, Kawauchi J, et al. ATF3 inhibits doxorubicin-induced apoptosis in cardiac myocytes: a novel cardioprotective role of ATF3. J Mol Cell Cardiol. 2002; 34:1387–1397. PMID: 12392999.

12. Inoue A, Yoshida N, Omoto Y, Oguchi S, Yamori T, Kiyama R, et al. Development of cDNA microarray for expression profiling of estrogen-responsive genes. J Mol Endocrinol. 2002; 29:175–192. PMID: 12370120.

13. Duckett JW. Walsh PC, Retik AB, Vaughan ED, editors. Hypospadias. Campbell's urology. 1998. 7th ed. Philadelphia: Saunders;p. 2093–2119.
14. Utsch B, Albers N, Ludwig M. Genetic and molecular aspects of hypospadias. Eur J Pediatr Surg. 2004; 14:297–302. PMID: 15543478.

15. Liu B, Wang Z, Lin G, Agras K, Ebbers M, Willingham E, et al. Activating transcription factor 3 is up-regulated in patients with hypospadias. Pediatr Res. 2005; 58:1280–1283. PMID: 16306208.

16. Beleza-Meireles A, Töhönen V, Söderhäll C, Schwentner C, Radmayr C, Kockum I, et al. Activating transcription factor 3: a hormone responsive gene in the etiology of hypospadias. Eur J Endocrinol. 2008; 158:729–739. PMID: 18426833.

17. Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002; 4:45–61. PMID: 11882781.

18. van der Zanden LF, van Rooij IA, Feitz WF, Vermeulen SH, Kiemeney LA, Knoers NV, et al. Genetics of hypospadias: are single-nucleotide polymorphisms in SRD5A2, ESR1, ESR2, and ATF3 really associated with the malformation? J Clin Endocrinol Metab. 2010; 95:2384–2390. PMID: 20215396.
19. Radpour R, Rezaee M, Tavasoly A, Solati S, Saleki A. Association of long polyglycine tracts (GGN repeats) in exon 1 of the androgen receptor gene with cryptorchidism and penile hypospadias in Iranian patients. J Androl. 2007; 28:164–169. PMID: 16957138.

20. Ban S, Sata F, Kurahashi N, Kasai S, Moriya K, Kakizaki H, et al. Genetic polymorphisms of ESR1 and ESR2 that may influence estrogen activity and the risk of hypospadias. Hum Reprod. 2008; 23:1466–1471. PMID: 18375409.

FIG. 1
Activating transcription factor 3 (ATF3) immunoreactivity in hypospadic and control tissues (×100). (A) There is no ATF3 immunoreactivity in circumcision control tissue. (B) ATF3 is clearly expressed in the nucleous of the cell (arrow) in the sc tissue of subcoronal hypospadic sample. (C) ATF3 protein localized in the nuclei of stromal cells (black arrow) and the vascular endothelium (white arrow) in sc tissue of mid penil hypospadic sample.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download