Abstract
Purpose
To evaluate the efficacy and safety of laparoscopic renal cryoablation (LRC) of small endophytic renal cell carcinoma, for which surgical treatment is technically difficult.
Materials and Methods
We enrolled patients with endophytic tumors from a prospectively collected database of 45 renal tumors in 39 patients who had undergone LRC from June 2005 to May 2009. An endophytic tumor was defined as less than 40% of the lesion extending off the surface of the kidney. We evaluated surgical and oncological outcomes.
Results
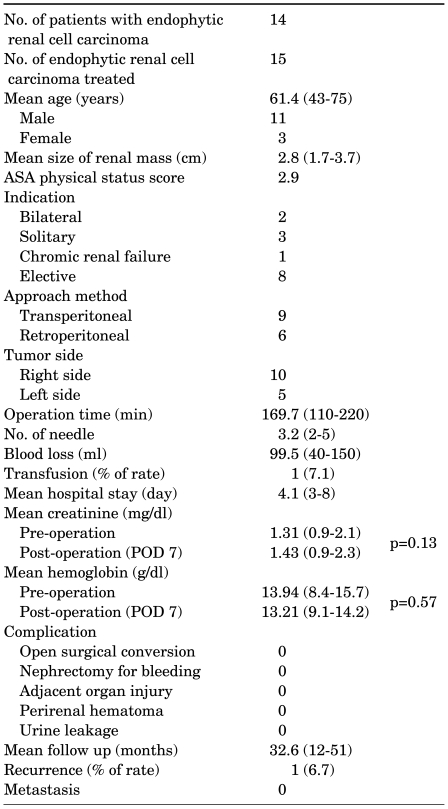
Among the treated tumors, 17 tumors (37.8%) were defined as endophytic tumors and 15 tumors from 14 patients were confirmed as renal cell carcinoma (RCC) in the pathologic examination of the tissue biopsy that was conducted at the time of LRC. The mean American Society of Anesthesiologists (ASA) score of the whole patient group was 2.9 (range, 1-4), and 85.7% (12/14) of the patients had an ASA physical status score over 3. The mean tumor size was 2.8 cm (range, 1.7-3.7 cm). The layout of the cryoprobe was carefully planned preoperatively on the basis of radiologic evaluation in all tumors. Multiple cryoprobes (mean, 3.2; range, 2-5) were used. No major complications, including open surgical conversion and nephrectomy due to bleeding, occurred. No patient experienced clinical symptoms of collecting system injuries. During the mean follow-up of 32.6 months (range, 12-51 months), radiologic evidence of tumor recurrence was found in one patient (6.7% for RCC). With the exception of this patient, all other patients have remained free of recurrence or metastasis, as determined by periodic radiologic workups.
Whereas the management of localized renal cell carcinoma (RCC) has evolved toward minimally invasive and nephron-sparing surgery, the treatment of endophytic renal tumors has remained problematic. These tumors typically require precise intracorporeal suturing and complex reconstruction, with the added time constraints imposed by renal ischemia. Even open partial nephrectomy is acknowledged to be technically demanding in this situation, with increased complication rates compared with procedures for tumors in peripheral locations [1]. In addition, serial studies of laparoscopic partial nephrectomy (LPN) report higher postoperative complication rates, which result in more ischemic time and longer hospital stays for endophytic tumors [2-4].
With the increasing application of minimally invasive surgery, several energy-based tissue ablation technologies including cryoablation and radiofrequency ablation are being investigated. In these procedures, tissue is destroyed in situ, thereby avoiding the complications induced by renal ischemia and surgical excision. Although energy-based ablation may injure the collecting system, clinical and preclinical studies on cryoablation have demonstrated the ability of this technique to manage tumors located almost within the collecting system without compromising the integrity of the underlying structure [5-7]. In addition, the laparoscopic approach allows the surgeon to position the cryoprobe by using intraoperative ultrasonography more precisely and to move adjacent organs away from the ablation site. If the surgical and oncological outcomes support the feasibility and efficacy of laparoscopic cryoablation (LRC) for endophytic renal tumors, and safety can be maintained during the surgical procedure, this method may provide an alternative nephron-sparing surgery for selected patients. To determine this, we evaluated the intermediate-term outcome of LRC in patients with endophytic RCC.
From June 2005 to May 2009, LRC was performed on 45 renal tumors in 39 patients at our institution. Indications for LRC included the presence of a localized, solid enhancing renal mass smaller than 4 cm in a patient at high risk for partial nephrectomy or older than 70 years of age [8]. High operative risk in our institution was defined as an American Society of Anesthesiology (ASA) physical status score of over 3 [9]. Among these inclusion criteria for LRC, absolute indications included bilateral tumors and a patient with a solitary kidney or renal insufficiency. A patient with normal contralateral renal function but poor operability was defined as an elective indication. All patients underwent a preoperative radiologic evaluation with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) in the case of renal insufficiency to delineate the parameters of the renal lesion, including location, size, depth, and position relative to hilar vessels and the collecting system.
All patients who underwent LRC in our institution were registered prospectively in a specific database that included all important information about tumor and patient characteristics including operative time, blood loss, hospital stay, pathology findings, and occurrence of complications. This allowed us to collect and analyze the data of the patients retrospectively, depending on the location of the tumor and pathologic findings. Three attending urologists reviewed the preoperative imaging studies for each patient to categorize the renal mass depending on tumor position. An endophytic location of a tumor in this study was defined as less than 40% of the lesion extending off the surface of the kidney (Fig. 1), as originally described by Finley et al [10]. Among all patients who underwent LRC, 15 tumors from 14 patients who were confirmed as having RCC by intraoperative needle biopsy and were followed up minimally for 12 months were exclusively enrolled in this series.
Patients were initially evaluated at 1 month and 3 months, then every 3 months during the first year. They were evaluated every 6 months during the second year and then annually. Follow-up evaluations involved a medical history update, physical examination, blood pressure measurement, contrast-enhanced CT or MRI, chest radiography, measurement of serum electrolytes, liver function tests, and renal function tests. A lack of enhancement on CT or MRI along with stable or decreased tumor size were considered signs of successful treatment. Recurrence was defined as increasing tumor size or lack of tumor shrinkage, with image enhancement [11].
All LRC procedures were conducted by a single surgeon with use of nearly identical technique. For the laparoscopic procedure, a standard technique using three ports was performed. In general, tumors anterior to a horizontal line within the coronal plane through the renal hilum were approached transperitoneally, and tumors posterior to this line were approached retroperitoneally. Real-time intraoperative ultrasonography (IOUS: Aloka Dynaview II, Americanlab, Miami, FL, USA) was used in all cases to identify the lesion and to determine the degree of the ice ball extension. The kidney was mobilized and the Gerota's fascia was opened to facilitate identification of the tumor. The fat overlying the tumor was placed aside and later retrieved for pathology examination. Up to two needle biopsies were taken from the tumor before the insertion of a cryoprobe. Then 1.47 mm cryoprobes (IceRod, Oncura, Plymouth Meeting, PA, USA) were inserted, each of which induced a -40℃ isothermal lesion 14.5x34 mm in diameter and radiating from the ablation probe. Before insertion into the kidney, the proper number and positions of the cryoprobes were carefully calculated on the basis of the preoperative imaging study and IOUS. Temperature probes were then inserted into the deep margins of the tumor. In all cases, a double-freeze cycle was applied, with an intervening thawing process. At the base of a lesion in proximity to the collecting system, the extent of the ice ball was identified by IOUS as a complete loss of echogenic lesion in the circumferential area of the base. Hemostasis was achieved by filling the probe tract with fibrin glue (Baxter, Deerfield, Il) and Surgicel (Johnson & Johnson, Irvine, CA, USA) after the second thaw allowed the safe removal of the cryoprobe.
Among 45 renal tumors treated with LRC in our institution, 17 (37.8%) were defined as endophytic tumors. Among them, 15 tumors from 14 patients were confirmed as RCCs and were exclusively enrolled in this series. Five tumors were centrally located, which was defined as being completely surrounded by normal parenchyma [12], with no visual clue regarding the location of the tumor on laparoscopy. One patient had a hilar tumor, which was defined as a tumor positioned medially within 5 mm of the renal artery or vein [13].
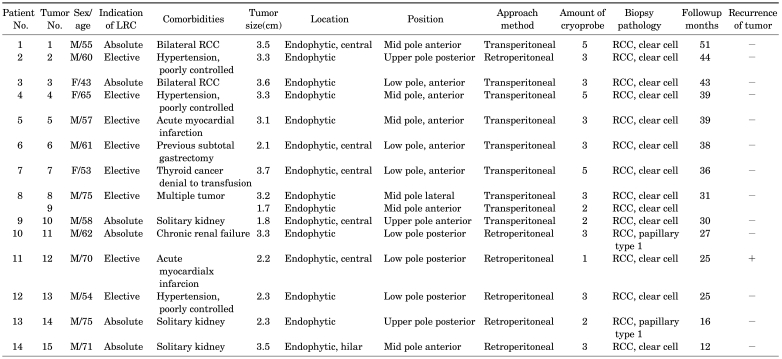
The patient demographics, perioperative characteristics, and outcomes are summarized in Table 1. The mean (range) age of the 14 enrolled patients was 61.4 years (range, 43-75 years) old. Four patients (28.6%) were over 70 years old, and their mean age was 72.8 years (range, 70-75 years) old with a mean ASA physical status score of 2.3 (range, 1-3). The other 10 patients had ASA physical status scores over 3. The mean ASA physical status score of the whole patient group was 2.9 (range, 1-4), and 85.7% (12/14) of patients had an ASA physical status score over 3. The indications for nephron-sparing surgery included a bilateral tumor in 2 patients, solitary kidney tumors in 3 patients, and chronic renal failure in 1 patient. The perioperative outcome of each patient is shown in Table 2.
The mean tumor size was 2.8 cm (range, 1.7-3.7 cm) and the mean operating time was 169.7 minutes (range, 110-220 minutes). The mean time of the first freeze cycle was 7.9 minutes, and the mean of the second freeze cycle was 7.4 minutes. In the case of five central tumors, more fat tissue overlying the kidney was removed to achieve an adequate image for monitoring the position of the cryoprobe tip. In the case of a hilar tumor, the major renal vessels, ureter, and renal pelvis were displaced from the ice ball with vascular loops, and rolled gauze was placed into the renal hilum. In all patients, multiple cryoprobes were used, and the mean number of cryoprobes needed was 3.2 (range, 2-5). The mean blood loss, measured as the amount in the suction device, was 99.5 ml (range, 40-150 ml).
No major complications, including open surgical conversion and nephrectomy due to bleeding, occurred in any patient. The serum creatinine and hemoglobin levels checked 7 days after surgery showed no significant differences compared with preoperative levels. The average preoperative and postoperative creatinine levels were 1.31 mg/dl and 1.43 mg/dl, respectively (p=0.13). The average preoperative and postoperative hemoglobin levels were 13.94 g/dl and 13.21 g/dl, respectively (p=0.57). One patient who had a previous subtotal gastrectomy for early gastric adenocarcinoma needed a postoperative transfusion due to a low preoperative baseline hemoglobin level (8.4 g/dl). The mean hospital stay was 4.1 days (range, 3-8 days), and during this stay, no one experienced clinical signs or symptoms of collecting system injuries, including flank pain, fever, and leukocytosis. In the pathologic examination, all of the patients showed common clear cell carcinoma, RCC, except for two patients with papillary type 1. Eight tumors were Fuhrman grade 2, and the others were grade 3.
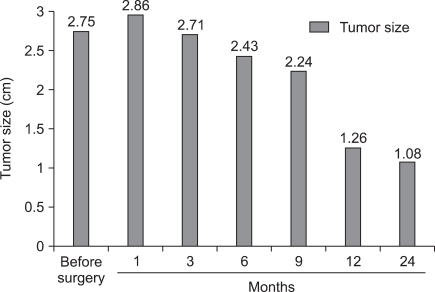
The mean follow-up was 32.6 months (range, 12-51 months). Follow-up imaging studies revealed no evidence of hydronephrosis, and all patients remained asymptomatic. Radiologic evidence of improper ablation was found in one patient (6.7%). A 2.2 cm sized central tumor was in direct contact with the collecting system, as shown by the enhancing portion in the deep peripheral area of the cryoablated site in an initial follow-up CT taken 1 month after LRC. Due to his high anesthesiologic risk from myocardial infarction, which required vascular stenting 3 times, and the anterior location of the tumor, which disturbs deployment of the probes for other minimally invasive ablative techniques including radiofrequency ablation, this patient is currently under active surveillance. Follow-up images showed no increase in the enhancing lesion or distant metastasis for 24 months after the initial LRC. Except for this patient, all other patients have remained free of recurrence or metastasis as shown by the periodic radiologic workups. The mean size of these properly treated lesions was 2.75±0.72 cm, 2.96±0.42 cm, 2.71±0.26 cm, 2.43±0.19 cm, 2.24±0.21 cm, 1.26±0.31 cm, and 1.08±0.34 cm before surgery, and at 1 month, 3 months, 6 months, 9 months, 12 months, and 24 months, respectively (Fig. 2, 3).
Because small renal tumors emerge incidentally during imaging for various indications, questions persist about the best way to treat endophytic tumors with a minimally invasive technique. Although 3-, 5-, and 10-year-old data encourage the use of renal cryoablation, especially for tumors in peripheral locations [14-16], extending these findings to endophytic tumors is still under debate. Because of the proximity to the renal vasculature and the collecting system, the cryoablation of an intraparenchymal endophytic tumor can potentially be disturbed by natural heat sinks, including the blood and urine. Actually, Wright et al reported two cases of treatment failure, both in endophytic tumors (i.e., completely intrarenal lesions), among 35 LRC lesions during an 18-month follow-up [17]. In the multivariate analysis in their study, only the endophytic status of a lesion predicted treatment failure. In contrast to this, Hurby et al reported the successful treatment by LRC in all 11 patients with tumors located within 5 mm of the renal vasculature during 11.3 months of follow-up [13]. Considering the technical difficulty of LPN in endophytic and hilar lesions, Nisbet et al formulated an algorithm on the basis of published series outcomes that recommended LRC for endophytic and LPN for exophytic and mesophytic lesions [18].
Our results provide additional evidence for the efficacy of LRC for the treatment of endophytic tumors. With intermediate-term follow-up, whereas 1 among 15 RCC lesions was managed improperly with LRC, the other lesions have remained free of recurrence or metastasis. Although we studied a small number of patients, the treatment failure rate of 6.7% in our series agrees with the positive margin rate of 1-7.4% in partial nephrectomy [19]. This result provides us with technical clues for successful ablation in cases of endophytic lesions. The first is the careful preoperative design of the layout of the cryoprobes. We usually planned the position of the cryoprobe tip on the basis of the preoperative imaging, including CT or MRI. In our series, the skin insertion site of each probe, the depth of insertion of the cryoprobe into the tumor in the operative filed, and the number of cryoprobes were decided on preoperatively on the basis of the shape, location, and diameter of the tumor. The sagittal and/or coronary sections of these images present additional information for this purpose. The IOUS image is also valuable, especially in guiding the location of the cryoprobe for endophytic tumors, because laparoscopic visual cues alone do not permit precise localization of cryoprobe tips, leaving IOUS as the only mode of real-time imaging. However, the image during freezing is distorted by several factors, including artifacts at the leading edge of the ice ball and inability to monitor the periphery of the mass, as noted by Badger et al [20]. In our LRC experience with endophytic tumors, the capacity of IOUS to monitor ice ball expansion is limited, especially for endophytic intraparenchymal tumors, because the collecting system itself behaves as an anechoic lesion.
To inflict lethal freezing injury throughout the tumor volume while sparing normal healthy tissues, the targeted lesion should be located within the lethal area of cryoablation, and a temperature below -40℃ is usually required to effectively destroy malignant renal tissue [21]. Unfortunately, no tool currently available can precisely define the three-dimensional configuration of this critical isothermal surface during cryoablation. Thus, we surmise that the use of multiple cryoprobes is more practical than focusing on the accuracy of the cryoprobe itself for conducting LRC for an endophytic lesion. Hypothetically, this approach might increase freezing efficiency by extending the coldest isothermal line, as compared with a single probe, and the distribution of the probes across the tumor might also compensate for an asymmetric tumor shape. Particularly for an intraparenchymal tumor, the use of multiple cryoprobes may have the additional advantage of evading the natural heat sink effect, a barrier intrinsic in low-temperature-based ablation.
One challenge a surgeon faces in a partial nephrectomy, whether open or laparoscopic, on a patient with an endophytic renal tumor is reconstruction of the collecting system. Cryoablation averts this challenge, but not the risk of injury to the collecting system, with resultant urine leakage, particularly for endophytic and deep lesions. However, recent reports on renal cryoablation support the tolerance of the collecting system to the radiographic ice ball formed in the procedure. Targeted renal pelvic cryoablation resulted in no urinary extravasations from a total of 15 lesions in a swine model [5]. In a clinical setting, six patients with intraparenchymal tumors who were treated by percutaneous cryoablation revealed no clinical evidence of ureteral sequelae [6]. In a comparison of 11 LRC and 12 LPN procedures for hilar tumors that were defined as located within 5 mm of the renal vasculature, the LRC group experienced no complications, whereas the complication rate of the LPN group was 50%, with urine leakage being the most significant complication [13]. Similarly, in our series of 14 patients with endophytic tumors, none showed clinical or radiological evidence of collecting system injury during the minimum follow-up of 28 months. These studies together support the safety of the cryoablative ice ball for the underlying structure of the kidney.
We still recognize several limitation of this series, including this being the experience of a single institution for a relatively small number of cases. The noncomparative, retrospective study design may also limit the application of our findings. Obviously, to establish the effectiveness of a new technique, prospective studies must be conducted to compare the new technique with conventional methods. However, it deserves notice that our patient criteria for LRC included high risk for anesthesia with severe co-morbidity or relatively old age, leaving LRC as the last surgical treatment option. Still, the application of cryoablation to renal tumors is currently in the investigational stage, despite the promising reported clinical outcomes. Therefore, although our data showed the intermediate-term oncologic efficacy of LRC, longer follow-up with larger scale series is still needed before ablative technologies can be established as a valid alternative option for the treatment of renal tumors and before their indication can be expanded to endophytic renal tumors.
In this series with an intermediate-term follow-up, LRC for small endophytic tumors showed acceptable oncological and surgical outcomes without adverse effects in the collecting system. These results also emphasize that careful preoperative design of the layout of the cryoprobes and the use of multiple cryoprobes should be given special consideration, especially in endophytic cases. However, further studies with longer term follow-up and larger patient groups are needed to establish the position of ablative technologies in the treatment of renal tumors and to extend their application to renal tumors in endophytic locations.
References
1. Hafez KS, Novick AC, Butler BP. Management of small solitary unilateral renal cell carcinomas: impact of central versus peripheral tumor location. J Urol. 1998; 159:1156–1160. PMID: 9507821.

2. Frank I, Colombo JR Jr, Rubinstein M, Desai M, Kaouk J, Gill IS. Laparoscopic partial nephrectomy for centrally located renal tumors. J Urol. 2006; 175:849–852. PMID: 16469563.

3. Venkatesh R, Weld K, Ames CD, Figenshau SR, Sundaram CP, Andriole GL, et al. Laparoscopic partial nephrectomy for renal masses: effect of tumor location. Urology. 2006; 67:1169–1174. PMID: 16765174.

4. Reisiger K, Venkatesh R, Figenshau RS, Bae KT, Landman J. Complex laparoscopic partial nephrectomy for renal hilar tumors. Urology. 2005; 65:888–891. PMID: 15882717.

5. Brashears JH 3rd, Raj GV, Crisci A, Young MD, Dylewski D, Nelson R, et al. Renal cryoablation and radio frequency ablation: an evaluation of worst case scenarios in a porcine model. J Urol. 2005; 173:2160–2165. PMID: 15879879.

6. Warlick CA, Lima GC, Allaf ME, Varkarakis I, Permpongkosol S, Schaeffer EM, et al. Clinical sequelae of radiographic iceball involvement of collecting system during computed tomography-guided percutaneous renal tumor cryoablation. Urology. 2006; 67:918–922. PMID: 16698352.

7. Cestari A, Guazzoni G, dell'Acqua V, Nava L, Cardone G, Balconi G, et al. Laparoscopic cryoablation of solid renal masses: intermediate term followup. J Urol. 2004; 172:1267–1270. PMID: 15371821.

8. Stein RJ, Kaouk JH. Renal cryotherapy: a detailed review including a 5-year follow-up. BJU Int. 2007; 99:1265–1270. PMID: 17441921.

9. Forrest JB, Rehder K, Cahalan MK, Goldsmith CH. Multicenter study of general anesthesia. III. Predictors of severe perioperative adverse outcomes. Anesthesiology. 1992; 76:3–15. PMID: 1729933.

10. Finley DS, Lee DI, Eichel L, Uribe CA, McDougall EM, Clayman RV. Fibrin glue-oxidized cellulose sandwich for laparoscopic wedge resection of small renal lesions. J Urol. 2005; 173:1477–1481. PMID: 15821463.

11. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005; 16:765–778. PMID: 15947040.

12. Black P, Filipas D, Fichtner J, Hohenfellner R, Thüroff JW. Nephron sparing surgery for central renal tumors: experience with 33 cases. J Urol. 2000; 163:737–743. PMID: 10687967.

13. Hruby G, Reisiger K, Venkatesh R, Yan Y, Landman J. Comparison of laparoscopic partial nephrectomy and laparoscopic cryoablation for renal hilar tumors. Urology. 2006; 67:50–54. PMID: 16413331.

14. Weld KJ, Figenshau RS, Venkatesh R, Bhayani SB, Ames CD, Clayman RV, et al. Laparoscopic cryoablation for small renal masses: three-year follow-up. Urology. 2007; 69:448–451. PMID: 17382142.

15. Berger A, Kamoi K, Gill IS, Aron M. Cryoablation for renal tumors: current status. Curr Opin Urol. 2009; 19:138–142. PMID: 19188767.

16. Aron M, Kamoi K, Haber GP, Desai MM, Canes D, Kaouk JH, et al. Laparoscopic renal cryoablation: long term oncologic outcomes with minimum 5- year follow-up. J Urol. 2008; 179(Suppl 1):209–210. abstract 596.
17. Wright AD, Turk TM, Nagar MS, Phelan MW, Perry KT. Endophytic lesions: a predictor of failure in laparoscopic renal cryoablation. J Endourol. 2007; 21:1493–1496. PMID: 18186689.

18. Nisbet AA, Rieder JM, Tran VQ, Williams SG, Chien GW. Decision tree for laparoscopic partial nephrectomy versus laparoscopic renal cryoablation for small renal masses. J Endourol. 2009; 23:431–437. PMID: 19265467.

19. Lam JS, Bergman J, Breda A, Schulam PG. Importance of surgical margins in the management of renal cell carcinoma. Nat Clin Pract Urol. 2008; 5:308–317. PMID: 18477995.

20. Badger WJ, de Araujo HA, Kuehn DM, Angresen KJ, Winfield HN. Laparoscopic renal tumor cryoablation: appropriate application of real-time ultrasonographic monitoring. J Endourol. 2009; 23:427–430. PMID: 19250024.

21. Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int. 2005; 95:1187–1191. PMID: 15892798.

FIG. 1
An example of an endophytic tumor. (A) The preoperative CT image showed a 2.8 cm well-enhanced mass in the right kidney at a distance of 2.6 mm (white arrow) from the collecting system. (B) At 18 months after surgery, the ablated region had diminished in size, and no region with enhancement was observed.
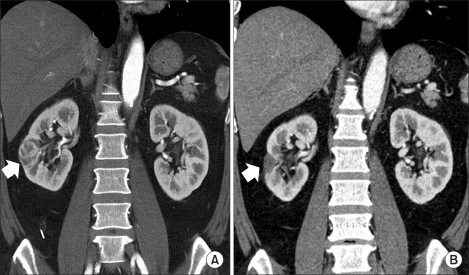
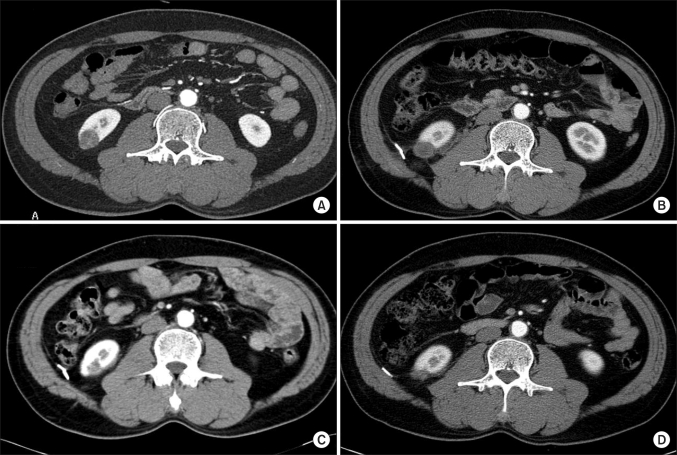
FIG. 2
Serial changes in a right endophytic tumor before and after cryoablation. (A) The preoperative CT image showed a 2.0 cm tumor mass encompassed by renal parenchyma in the lower pole of the right kidney. (B) Postoperative image at 3 months. (C) Postoperative image at 12 months. (D) In this image 24 months after surgery, the ablated lesion had nearly vanished.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download