Introduction
Metastatic involvement of the heart occurs in up to 18% of all patients with malignant tumors.1) There are few reports about the rates at which squamous cell carcinomas metastasize after standard surgical treatment with radiotherapy. Clinical presentation of cardiac metastasis can vary from silent to prominent heart failure symptoms, including pericardial lesion related symptomatology or even sudden death. Cardiac metastasis can evolve on a silent and chronic course that it can be discovered only after death. Since most cardiac metastases appear in patients of advanced stages of the malignancy, prognosis is generally poor and therapeutic options are limited. In patients with cancer, cardiac metastases are usually difficult to diagnose unless the patients don't complain of any related symptoms or in case that clinicians have no suspicion about them. We present an autopsy case that cardiac metastasis from oral cavity cancer brought a sudden death even though electrocardiographic abnormalities were identified before death.
Case Report
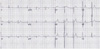
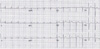
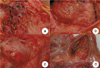
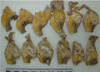
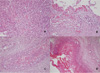
In September, a 60-year-old man with oral cancer visited a university hospital for checking up whether any tumor recurrence or distant metastasis occurred or not. However, the patient suddenly collapsed after 18 fluorodeoxyglucose (18F-FDG) was intravenously administered for PET-CT scanning. Despite of emergency resuscitation procedures for 2 hours, the patient was dead with no response. According to the given medical record, the patient's oral cancer had been diagnosed as an invasive squamous cell carcinoma with the mass size of 3.5 cm × 2.5 cm × 2.3 cm in the left retromolar trigone (T4N1M0) in February. At the time of this diagnosis, the patient's heart was unremarkable with normal sinus rhythm (HR 70 beats/min) in ECG (Fig. 1). The patient underwent a surgical operation with wide excision, left radical neck dissection, left marginal mandibulectomy and soft tissue reconstruction. He got through several cycles of radiotherapy from April to June and took otorhinolaryngology department follow-ups as an outpatient. A CT scan in September 1 revealed a recurrent lesion in the left medial pterygoid muscle and metastatic lesions in the lungs and mediastinal lymph nodes. At admission in September 6, for reoperation, the patient showed aching pain of the mentum area, leukocytosis (white blood cells 11,230/µl), and anemia (hemoglobin 9.2 g/dL). No abnormal feature was found in a physical examination about the patient's chest. The stethoscope examination finding described in the medical record didn't reveal any murmur, thrill or heaving. Fever up to body temperature 38.1℃ was developed in the next day. The ECG 09:42 at the admission day 3 showed sinus tachycardia (HR 104 beats/min) and low voltage in limb leads (Fig. 2). For a PET-CT scanning, 18F-FDG was intravenously administered to him at 18:42. However, the patient suddenly got lost his consciousness and vital signs several minutes later. Cardiopulmonary resuscitation procedures were performed but several intubation trials failed. Tracheostomy managed to be done 19:40. He didn't show any response despite of resuscitation 30 minutes more after tracheostomy was performed. On subsequent medicolegal autopsy, a tumor mass with a central necrosis was found along the right atrioventricular groove between the right atrial appendage and the right ventricle free wall. The gray-white tumor mass with the size of 4.8 cm × 4.5 cm × 3.5 cm was encircling the proximal portion of the right coronary artery. It was histologically compatible with well differentiated squamous cell carcinoma with infiltration of the pericardium. The right coronary artery and left coronary artery had mild to moderate degrees of atherosclerosis. Carcinoma cells focally infiltrated deep to the endothelium of the right coronary artery in which a focal thrombosis was present. Tumor emboli were also found in the anterior right atrial branch of the right coronary artery and a few small vessels near the sinoatrial and atrioventricular nodal tissues. The pericardium typically had the feature of chronic constrictive pericarditis that was pathologically due to tumor cell infiltration accompanying fibrinoid change (Figs. 3-5). Microscopically, several tumor emboli were found in the intrapulmonary vessels and the periaortic lymph nodes. Toxicologic study of the blood and gastric content was unremarkable. The cause of death was ruled as sudden cardiac death due to cardiac metastasis from oral squamous cell carcinoma.
Discussion
It is no wonder that any carcinoma including sarcomas can metastasize to the heart. Common tumors with cardiac metastasis potential are usually carcinomas of the lung, pleura, breast, esophagus, and kidney, and malignant lymphoma, leukemia, sarcoma, and malignant melanoma. Reported incidence of cardiac metastasis at autopsy ranges from 1.5% to 21.6% for all cancer patients and cardiac metastases have been in 0.2 - 6.5% of subjects in unselected autopsy series.2, 3) While cardiac metastasis from the tongue is consistently reported with some frequency, cardiac metastasis from oral squamous cell carcinoma including oral cavity malignant tumors seems relatively infrequent or rare for forensic pathologists to see in medicolegal autopsy practice. Cardiac metastases are surely much more common than primary cardiac tumors. But antemortem diagnosis of cardiac metastasis is known to be seldom made because more than 90% of cardiac metastasis patients are clinically silent.4) Cardiac metastasis does not generally lead to clinical findings at an early stage. At more advanced stages, metastatic tumors to the heart could gradually give rise to heart failure, conduction abnormalities, valvular disease resembling mitral stenosis or angina pain. However even advanced metastatic lesions to the heart can also be so silent until death that it is possible to die suddenly. Sudden death reportedly occurs in about 3% of patients with cardiac metastasis. This sudden cardiac death are thought to be related with massive invasion of the myocardium, coronary flow interference, distortion of the cardiac valves, intra-cardiac blood flow obstruction, rupture of the myocardium or disturbance of the rhythm. In addition, pericardial effusion related tamponade or large mass effect of the pericardial space also can contribute to.5) Therefore common clinical signs and symptoms of cardiac Death due metastasis become present as congestive heart failure, arrhythmias, electrocardiographic abnormalities and pericardial effusion. Among these signs and symptoms, ECG changes can be clinically an important clue for diagnosis of cardiac metastasis in patients with malignant tumors because it induces higher incidence of arrhythmias than those without metastasis. Although there are no specific ECG changes diagnostic for cardiac metastasis, ECG abnormalities in cardiac metastasis are reportedly tachy or brady arrhythmias, low voltage QRS complex, prolonged ST segment elevation without Q waves, non-specific ST-T wave changes, complete heart block above the bundle of His with a narrow QRS complex, etc.6) As regarding the death circumstance of the presenting case, the deceased presented ECG abnormalities 9 hours before sudden arrest. They were low QRS voltage and sinus tachycardia that were different from the ECG 7 months earlier. The heart and pericardium on autopsy revealed typical of chronic active fibrinoid pericarditis induced tumor cell infiltration, accompanied by a large metastatic mass in the right atrium appendage, extending to the left ventricle. It is sure that the antemortem ECG change of the deceased was definitely due to cardiac metastasis. The authors would like to cast some questions and suggestions to clinicians about this medicolegal autopsy case as follows : a) Was the patient's ECG change 9 hours before cardiac arrest so insignificant to compare with the previous ECG or proceed other cardiovascular evaluation like echocardiography? b) Is it clinically a frequent feature that stethoscopic auscultation findings are normal in the degree like what the deceased's heart and pericardium showed on autopsy? c) Wasn't the airway securing for resuscitation too late for the deceased, considering that tracheostomy was done nearly 50 minutes after sudden arrest through several intubation fails. The presenting medicolegal autopsy case underlies that sudden death due to cardiac metastasis from primary oral squamous cell carcinoma diagnosed 7 months earlier can occur although the primary tumor had been treated curatively with standard treatment of wide otorhinolaryngological resection and radiotherapy. In addition, although given the non-specific and diverse nature of clinical manifestations, a high level of diagnostic suspicion about cardiac metastasis is required if there are ECG changes during follow-ups in patient with primary malignant tumor. Cardiac metastasis is usually detected in advanced stages of primary malignant tumor such that the outcome is generally poor in the majority of cases. Therefore the main object of surgical resection, chemotherapy and radiotherapy is to relieve life-threatening symptoms or prevent sudden death.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download