Abstract
Objecti
The aim of this study was to clarify whether stimulation of recombinant IL-17, TLR2 and TLR4 by their specific ligands induces the production of RANKL and IL-6 in the fibroblast-like synoviocytes (FLSs) from RA patients.
Methods
FLSs were isolated from RA synovial tissues and they were stimulated with the IL-17, TLR2 ligand bacterial peptidoglycan (PGN) and TLR4 ligand lipopolysaccharide (LPS). The RANKL levels were assessed by RT-PCR and western blotting. The expressions of IL-17, TLR2, TLR4, RANKL and IL-6 in the RA synovium were quantified by immunohistochemistry and these values were compared with the values obtained in the osteoarthritis synovium. The increased IL-6 production in the culture supernatants of the RA FLSs was quantified by sandwich ELISA.
Go to : 
REFERENCES
1). Lacey D., Sampey A., Mitchell R., Bucala R., Santos L., Leech M, et al. Control of fibroblast-like synoviocyte proliferation by macrophage migration inhibitory factor. Arthritis Rheum. 2003. 48:103–9.

2). Miossec P. An update on the cytokine network in rheumatoid arthritis. Curr Opin Rheumatol. 2004. ;16;218-22.

3). Chabaud M., Durand JM., Buchs N., Fossiez F., Page G., Frappart L, et al. Human interleukin-17: a T cell-derivedproinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999. 42:963–70.
4). Sato K., Suematsu A., Okamoto K., Yamaguchi A., Morishita Y., Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006. 203:2673–82.

5). Aggarwal S., Ghilardi N., Xie MH., de Sauvage FJ., Gurney AL. Interleukin-23 promotes a distinct CD4T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003. 278:1910–4.
6). Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003. 48:594–601.

7). Bettelli E., Carrier Y., Gao W., Korn T., Strom TB., Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 andregulatory T cells. Nature. 2006. 441:235–8.
8). Mangan PR., Harrington LE., O'Quinn DB., Helms WS., Bullard DC., Elson CO, et al. Transforming growth factor-beta induces development of the T (H)17 lineage. Nature. 2006. 441:231–4.
9). Fantini MC., Rizzo A., Fina D., Caruso R., Becker C., Neurath MF, et al. IL-21 regulates experimental colitis by modulating the balance between T (reg) and Th17 cells. Eur J Immunol. 2007. 37:3155–63.
10). Nakashima T., Kobayashi Y., Yamasaki S., Kawakami A., Eguchi K., Sasaki H, et al. Protein expression and functional difference of modulation of the expression by osteotropic factors and cytokines. Biophys Res Commun. 2000. 275:768–75.
11). LeGrand A., Fermor B., Fink C., Pisetsky DS., Weinberg JB., Vail TP, et al. Interleukin-1, tumor necrosis factor a, and Interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001. 44:2078–83.
12). Attur MG., Patel RN., Abramson SB., Amin AR. Interleukin-17 up-regulation of nitric oxide production in human osteoarthritis cartilage. Arthritis Rheum. 1997. 40:1050–3.

13). Cai L., Yin JP., Starovasnik MA., Hogue DA., Hillan KJ., Mort JS, et al. Pathways by which interleukin 17 induces articular cartilage breakdown in vitro and in vivo. Cytokine. 2001. 16:10–21.

14). Kotake S., Udagawa N., Takahashi N., Matsuzaki K., Itoh K., Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999. 103:1345–52.

15). Lee JH., Cho ML., Kim JI., Moon YM., Oh HJ., Kim GT, et al. Interleukin 17 (IL-17) Increases the Expression of Toll-like Receptor-2, 4, and 9 by Increasing IL-1bT and IL-6 Production in Autoimmune Arthritis. J Rheumatol. 2009. 36:684–92.
16). Brentano F., Kyburz D., Schorr O., Gay R., Gay S. The role of Toll-like receptor signalling in the pathogenesis of arthritis. Cell Immunol. 2005. 203:90–6.

17). Seibl R., Birchler T., Loeliger S., Hossle JP., Gay RE., Saurenmann T, et al. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003. 162:1221–7.

18). Madhok R., Crilly A., Watson J., Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993. 52:232–4.

19). Nishimoto N., Kishimoto T. Inhibition of IL-6 for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004. 4:386–91.

20). Tamura T., Udagawa N., Takahashi N., Miyaura C., Tanaka S., Yamada Y, et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin-6. Proc Natl Acad Sci. 1993. 90:11924–8.
21). Chole RA. Cellular and subcellular events of bone resorption in human and experimental cholesteatoma: The role of osteoclasts. Ann Otol Rhinol Laryngol. 1984. 94:76–95.

22). Kong YY., Yoshida H., Sarosi I., Tan HL., Timms E., Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lympocyte development and lymphnode organogenesis. Nature. 1999. 397:315–23.
23). Takahashi N., Udagawa N., Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999. 256:449–55.

24). Haynes DR., Crotti TN., Potter AE., Loric M., Atkins DW., Findlay DM. The osteoclastogenic molecules RANKL and RANK are associated with periprosthetic osteolysis. J Bone Joint Surg (Br). 2001. 83:902–11.

25). Kudo O., Sabokbar A., Pocock A., Itonaga I., Fujikawa Y., Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL independent mechanism. Bone. 2003. 32:1–7.
26). Madhok R., Crilly A., Watson J., Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993. 52:232–4.

27). McFarland HF., Martin R. Multiple sclerosis: acomplicated picture of autoimmunity. Nat Immunol. 2007. 8:913–9.
28). Toh ML., Miossec P. The role of T cells in rheumatoid arthritis: new subsets and new targets. Curr Opin Rheumatol. 2007. 19:284–8.

29). Chevrel G., Page G., Granet C., Streichenberger N., Varennes A., Miossec P. Interleukin-17 increases the effects of IL-1 beta on muscle cells: arguments for the role of T cells in the pathogenesis of myositis. J Neuroimmunol. 2003. 137:125–33.
30). Page G., Chevrel G., Miossec P. Anatomic localization of immature and mature dendritic cell subsets in dermatomyositis and polymyositis. Arthritis Rheum. 2004. 50:199–208.
31). Kim KW., Cho ML., Lee SH., Oh HJ., Kang CM., Ju JH, et al. Human rheumatoid synovial fibroblasts promote osteoclastogenic activity by activating RANKL via TLR-2 and TLR-4 activation. Immunol Lett. 2007. 110:54–64.

32). Sack U., Kinne RW., Marx T., Heppt P., Bender S., Emmrich F. Interleukin-6 in synovial fluid is closely associated with chronic synovitis in rheumatoid arthritis. Rheumatol Int. 1993. 13:45–51.

33). Hwang SY., Kim JY., Kim KW., Park MK., Moon YM., Kim WU, et al. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kPB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther. 2004. 6:R120–8.
34). Kurihara N., Bertolini D., Suda T., Akiyama Y., Roodman GD. IL-6 stimulates osteoclast-like multinucleated cell formation in long term human marrow cultures by inducing IL-1 release. The Journal of Immunology. 1990. 144:4226–30.
Go to : 
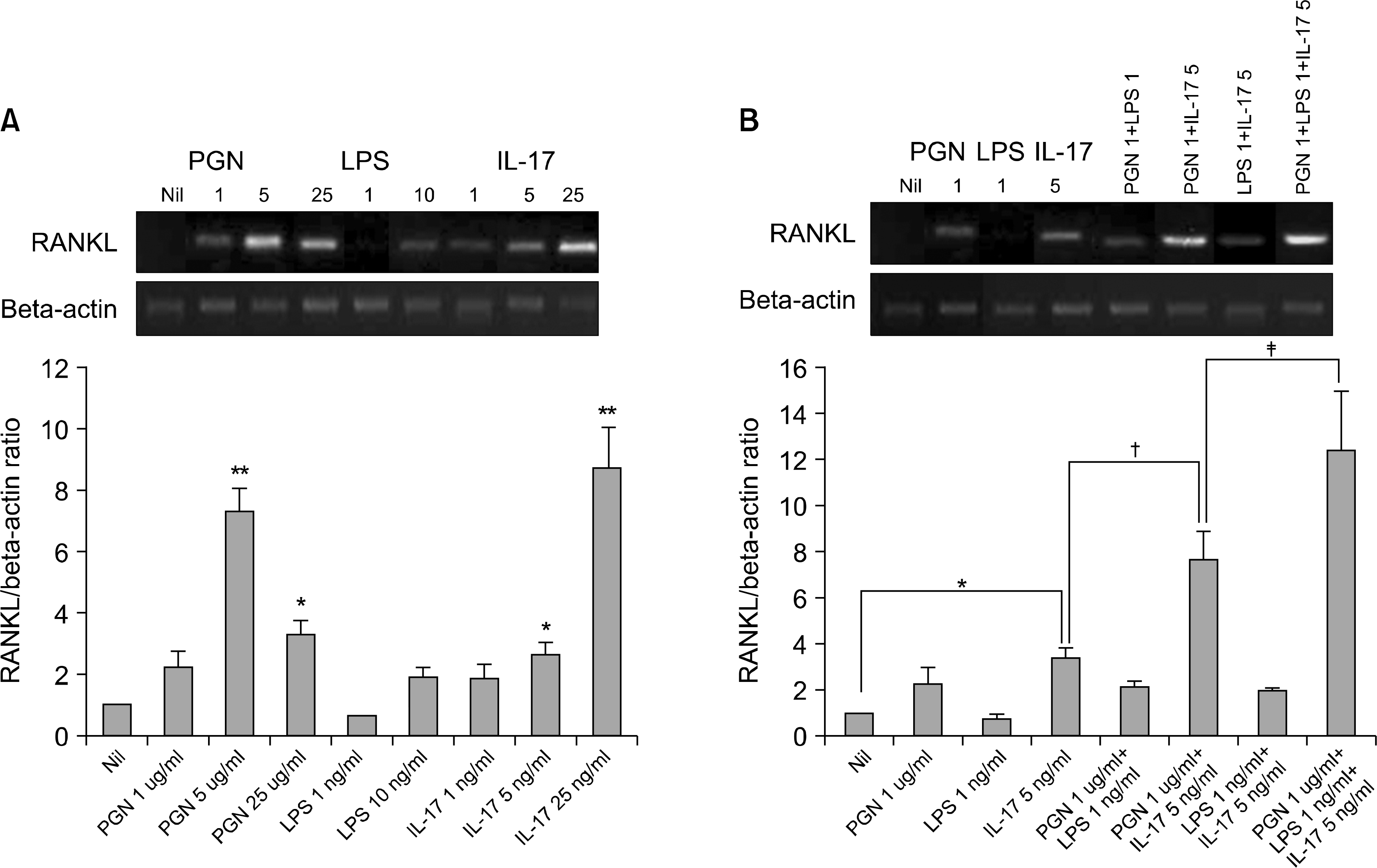 | Fig. 1.RANKL mRNA expression by the RA-FLSs after stimulation with IL-17, PGN and LPS (A) The RANKL mRNA expression induced by IL-17, PGN and LPS. The FLSs were cultured with IL-17 (1, 5, 25 ng/ml), PGN (1, 5, 25 ng/ml) and LPS (1, 10 ng/ml) for 72 h. The expression of RANKL mRNA was evaluated by RT-PCR. The optical density was normalized to the band for beta-actin. (B) The RANKL mRNA expression induced by IL-17, PGN, LPS, IL-17 plus PGN, IL-17 plus LPS, PGN plus LPS, and IL-17 plus PGN plus LPS. The expression of RANKL mRNA was evaluated by RT-PCR. ∗p<0.05, ∗∗p<0.01 compared with Nil, †<0.05 compared with IL-17 5 ng/ml, ‡P<0.05 compared with PGN 1 ug/ml +IL-17 5 ng/ml. |
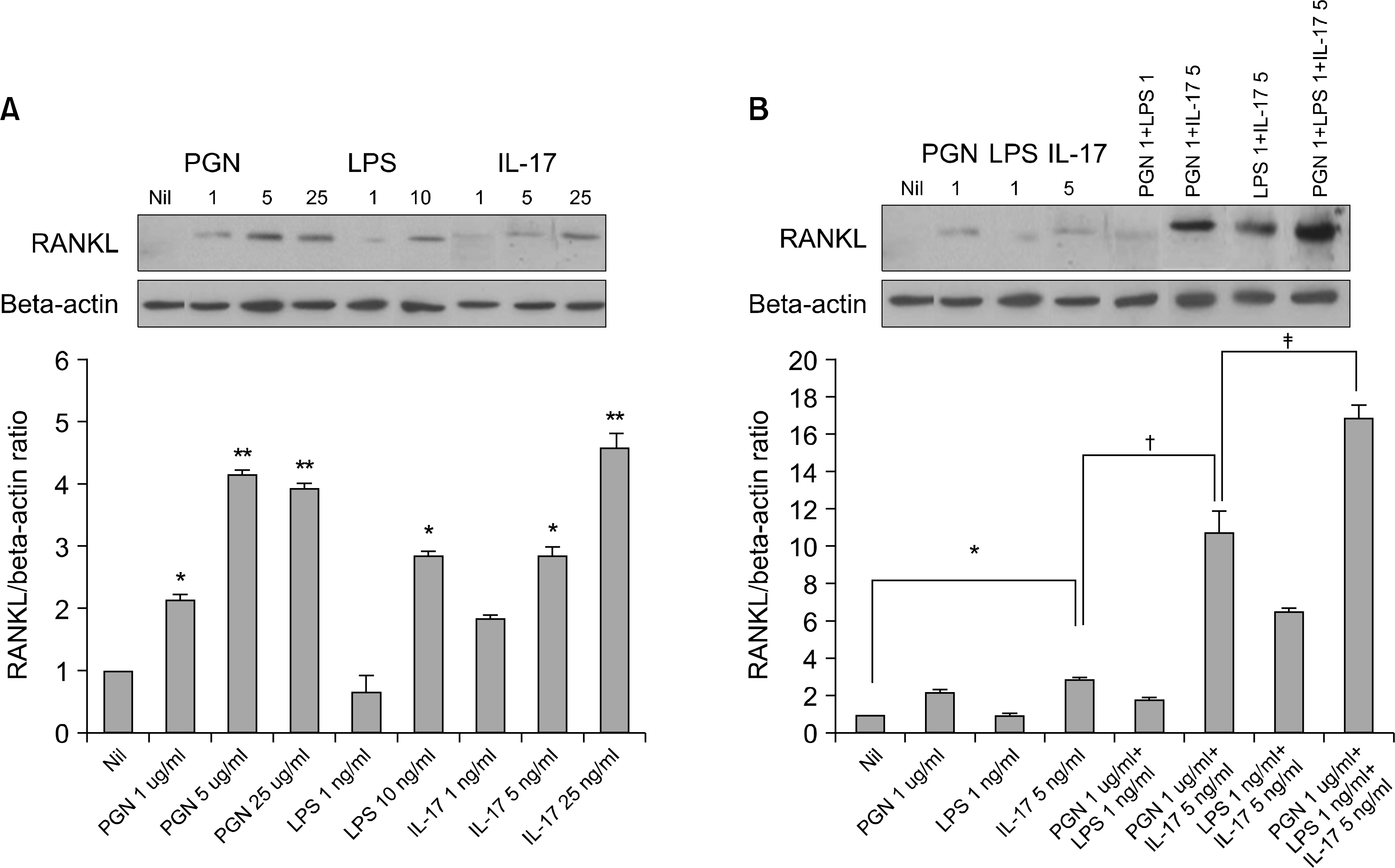 | Fig. 2.The RANKL protein expression in the RA-FLSs increased significantly after treatment with IL-17, PGN and LPS (A) The RA-FLSs were cultured with or without IL-17 (1, 5, 25 ng/ml), PGN (1, 5, 25 ng/ml) or LPS (1, 10 ng/ml) for 72 h. The RANKL protein expression was measured in the cell lysates by western blot analysis using goat polyclonal anti-RANKL antibody. (B) The RANKL protein expression induced by IL-17, PGN, LPS, IL-17 plus PGN, IL-17 plus LPS, PGN plus LPS, and IL-17 plus PGN plus LPS. The results are expressed as the ratio of the densitometric intensity of the RANKL product to that of the beta-actin product. The results are presented as the mean±S.E.M., n=3. ∗p<0.05, ∗∗p<0.01 compared with Nil, †p<0.05 compared with IL-17 5 ng/ml, ‡p<0.05 compared with PGN 1 ug/ml +IL-17 5 ng/ml. |
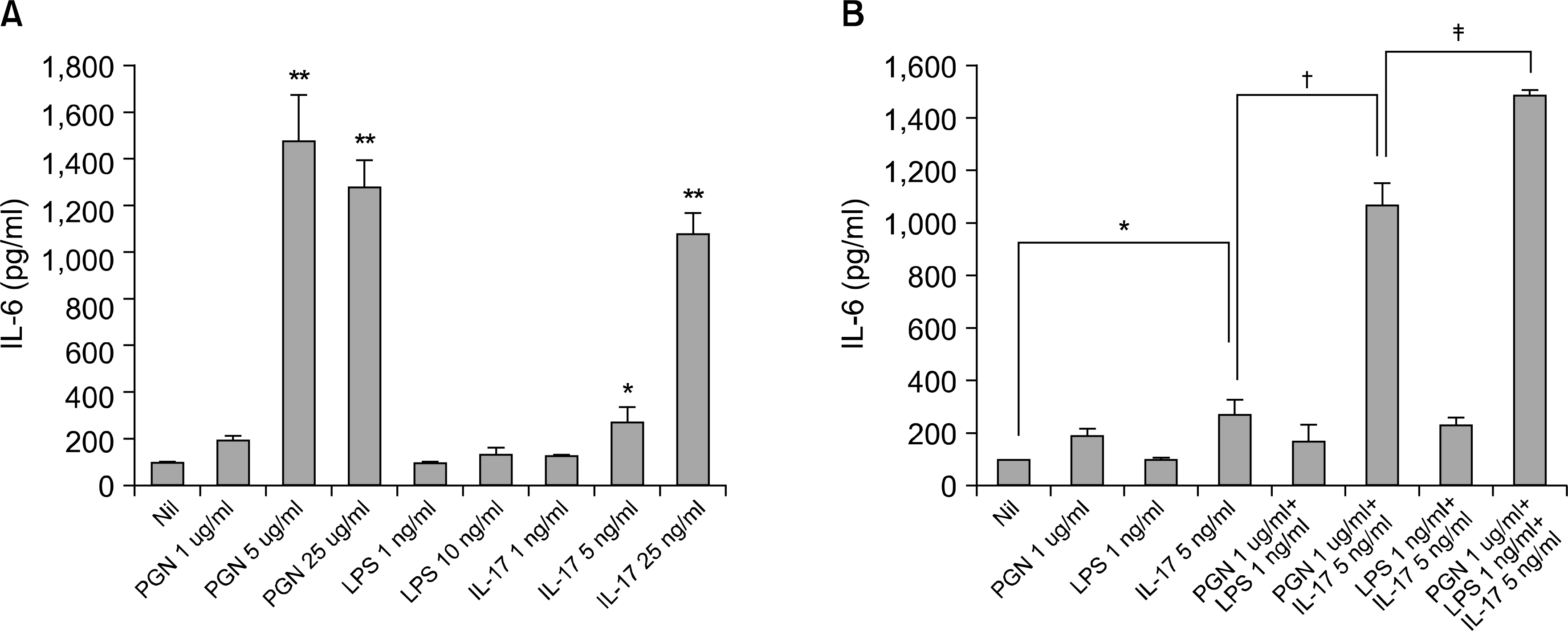 | Fig. 3.Quantitation of the IL-6 production by IL-17, PGN and LPS in the RA-FLSs. (A) The FLSs were cultured with IL-17 (1, 5, 25 ng/ml), PGN (1, 5, 25 ng/ml) or LPS (1, 10 ng/ml) for 72 h, and the IL-6 concentrations in the culture supernatants were determined by enzyme-linked immunosorbent assay. (B) The IL-6 production induced by IL-17, PGN, LPS, IL-17 plus PGN, IL-17 plus LPS, PGN plus LPS, and IL-17 plus PGN plus LPS. Values are the mean and SEM of triplicate cultures. ∗p<0.05, ∗∗p<0.01 compared with Nil, †p<0.05 compared with IL-17 5 ng/ml, ‡p<0.05 compared with PGN 1 ug/ml +IL-17 5 ng/ml. |
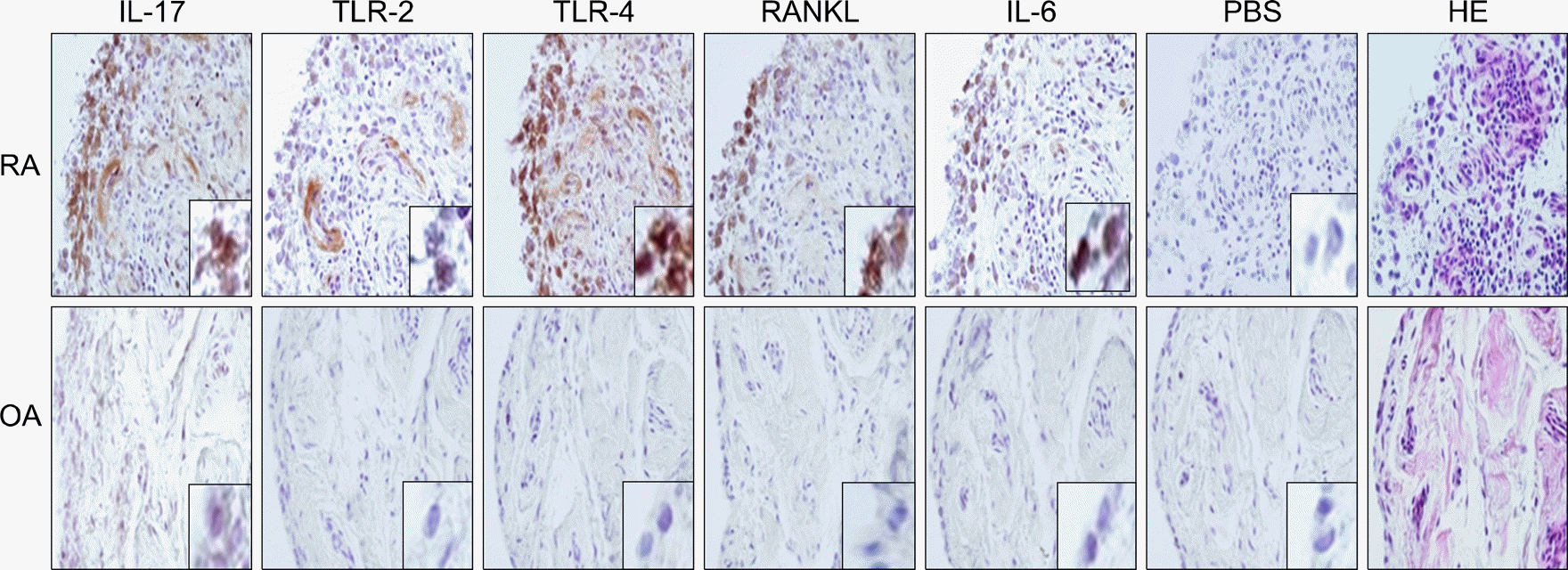 | Fig. 4.The expressions of IL-17, TLR-2, TLR-4, RANKL and IL-6 was significantly greater in the RA synovium than those in the OA synovium. Immunohistochemical detection of IL-17, TLR-2, TLR-4, RANKL and IL-6 in the synovium of patients with RA and OA. All the tissues were counterstained with hematoxylin (original magnification, ×400). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download