Abstract
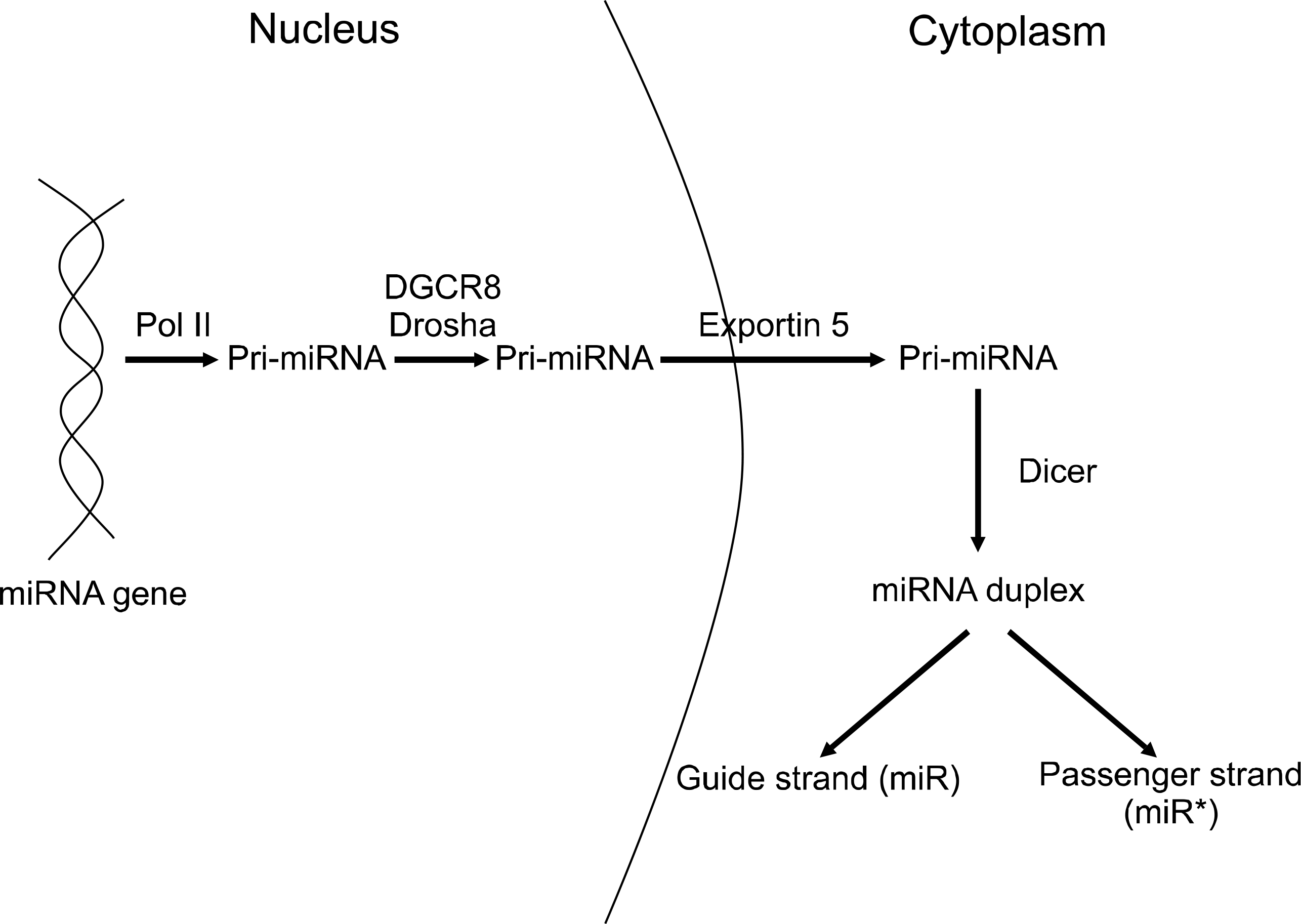
MicroRNAs (miRNAs) are small, single-stranded, non-coding RNA molecules of 20~22 nucleotides, which are involved in many biologic functions such as development, cell proliferation, differentiation, and apoptosis. In addition to these biologic functions, recent reports have demonstrated that miRNAs play important roles in the development of the immune system and the regulation of immune responses. Dysregulation of miRNAs might be involved in the pathogenesis of autoimmune diseases such as rheumatoid arthritis (RA). Recent studies have shown that miR-146a, miR-155, and miR-203 are overexpressed in RA and that miR-124a is under expressed in RA. miR-146 downregulates the expression of IL-1 receptor associated kinase 1 and TNF receptor-associated factor 6 involved in IL-1β signaling, and miR-155 suppresses the expression of matrix metalloproteinases 1 and 3, suggesting that these miRNAs act as negative feedback regulators of inflammation and tissue damage in RA. In this report, we review the current knowledge about miRNAs and summarize the involvement of miRNAs in RA.
REFERENCES
1). Pauley KM., Cha S., Chan EK. Microrna in autoimmunity and autoimmune diseases. J Autoimmun. 2009. 32:189–94.

2). Furer V., Greenberg JD., Attur M., Abramson SB., Pillinger MH. The role of microrna in rheumatoid arthritis and other autoimmune diseases. Clin Immunol. 2010. 136:1–15.

3). Sonkoly E., Pivarcsi A. Advances in micrornas: Implications for immunity and inflammatory diseases. J Cell Mol Med. 2009. 13:24–38.

4). Sonkoly E., Wei T., Janson PC., SaUaUf A., Lundeberg L., Tengvall-Linder M, et al. Micrornas: novel regulators involved in the pathogenesis of psoriasis? PLoS One. 2007. 2:e610.

5). Alevizos I., Illei GG. MicroRNAs in Sjogren's syndrome as a prototypic autoimmune disease. Autoimmun Rev. 2010. 9:618–21.
6). Dai Y., Huang YS., Tang M., Lv TY., Hu CX., Tan YH, et al. Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus. 2007. 16:939–46.

7). Tang Y., Luo X., Cui H., Ni X., Yuan M., Guo Y, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009. 60:1065–75.

8). Lee RC., Feinbaum RL., Ambros V. The c. Elegans heterochronic gene lin-4 encodes small rnas with anti-sense complementarity to lin-14. Cell. 1993. 75:843–54.

9). Pasquinelli AE., Reinhart BJ., Slack F., Martindale MQ., Kuroda MI., Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory rna. Nature. 2000. 408:86–9.

10). Han J., Pedersen JS., Kwon SC., Belair CD., Kim YK., Yeom KH, et al. Posttranscriptional crossregulation between drosha and dgcr8. Cell. 2009. 136:75–84.

11). Lee Y., Jeon K., Lee JT., Kim S., Kim VN. Microrna maturation: stepwise processing and subcellular localization. EMBO J. 2002. 21:4663–70.

12). Ro S., Park C., Young D., Sanders KM., Yan W. Tissue-dependent paired expression of mirnas. Nucleic Acids Res. 2007. 35:5944–53.

13). Ruby JG., Jan CH., Bartel DP. Intronic microrna precursors that bypass drosha processing. Nature. 2007. 448:83–6.

14). Filipowicz W., Bhattacharyya SN., Sonenberg N. Mechanisms of post-transcriptional regulation by micrornas: are the answers in sight? Nat Rev Genet. 2008. 9:102–14.

15). Vasudevan S., Tong Y., Steitz JA. Switching from repression to activation: micrornas can up-regulate translation. Science. 2007. 318:1931–4.

17). Tili E., Michaille JJ., Costinean S., Croce CM. Micrornas, the immune system and rheumatic disease. Nat Clin Pract Rheumatol. 2008. 4:534–41.

18). Bartel DP. Micrornas: genomics, biogenesis, mechanism, and function. Cell. 2004. 116:281–97.
19). Siomi H., Siomi MC. Posttranscriptional regulation of microrna biogenesis in animals. Mol Cell. 2010. 38:323–32.

20). Taganov KD., Boldin MP., Chang KJ., Baltimore D. Nf-kappab-dependent induction of microrna mir-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006. 103:12481–6.
21). Perry MM., Moschos SA., Williams AE., Shepherd NJ., Larner-Svensson HM., Lindsay MA. Rapid changes in microrna-146a expression negatively regulate the il-1beta-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008. 180:5689–98.
22). Hou J., Wang P., Lin L., Liu X., Ma F., An H, et al. Microrna-146a feedback inhibits rig-i-dependent type i ifn production in macrophages by targeting traf6, irak1, and irak2. J Immunol. 2009. 183:2150–8.

23). Motsch N., Pfuhl T., Mrazek J., Barth S., Grasser FA. Epstein-barr virus-encoded latent membrane protein 1 (lmp1) induces the expression of the cellular microrna mir-146a. RNA Biol. 2007. 4:131–7.

24). Nahid MA., Pauley KM., Satoh M., Chan EK. Mir-146a is critical for endotoxin-induced tolerance: implication in innate immunity. J Biol Chem. 2009. 284:34590–9.
25). Cobb BS., Hertweck A., Smith J., O'Connor E., Graf D., Cook T, et al. A role for dicer in immune regulation. J Exp Med. 2006. 203:2519–27.

26). Bi Y., Liu G., Yang R. Micrornas: novel regulators during the immune response. J Cell Physiol. 2009. 218:467–72.

27). Ceppi M., Pereira PM., Dunand-Sauthier I., Barras E., Reith W., Santos MA, et al. Microrna-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci USA. 2009. 106:2735–40.

28). O'Connell RM., Taganov KD., Boldin MP., Cheng G., Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007. 104:1604–9.
29). Tili E., Michaille JJ., Cimino A., Costinean S., Dumitru CD., Adair B, et al. Modulation of mir-155 and mir-125b levels following lipopolysaccharide/tnf-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007. 179:5082–9.
30). Martinez-Nunez RT., Louafi F., Friedmann PS., Sanchez-Elsner T. Microrna-155 modulates the pathogen binding ability of dendritic cells (dcs) by down-regulation of dc-specific intercellular adhesion molecule-3 grabbing non-integrin (dc-sign). J Biol Chem. 2009. 284:16334–42.

31). Rodriguez A., Vigorito E., Clare S., Warren MV., Couttet P., Soond DR, et al. Requirement of bic/microrna-155 for normal immune function. Science. 2007. 316:608–11.
32). Lu LF., Thai TH., Calado DP., Chaudhry A., Kubo M., Tanaka K, et al. Foxp3-dependent microrna155 confers competitive fitness to regulatory t cells by targeting socs1 protein. Immunity. 2009. 30:80–91.

33). Teng G., Hakimpour P., Landgraf P., Rice A., Tuschl T., Casellas R, et al. Microrna-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008. 28:621–9.

34). Vigorito E., Perks KL., Abreu-Goodger C., Bunting S., Xiang Z., Kohlhaas S, et al. Microrna-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007. 27:847–59.

35). Moschos SA., Williams AE., Perry MM., Birrell MA., Belvisi MG., Lindsay MA. Expression profiling in vivo demonstrates rapid changes in lung microrna levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics. 2007. 8:240.

36). Li QJ., Chau J., Ebert PJ., Sylvester G., Min H., Liu G, et al. Mir-181a is an intrinsic modulator of t cell sensitivity and selection. Cell. 2007. 129:147–61.

37). Alsaleh G., Suffert G., Semaan N., Juncker T., Frenzel L., Gottenberg JE, et al. Bruton's tyrosine kinase is involved in mir-346-related regulation of il-18 release by lipopolysaccharide-activated rheumatoid fibroblast-like synoviocytes. J Immunol. 2009. 182:5088–97.

38). Fulci V., Scappucci G., Sebastiani GD., Giannitti C., Franceschini D., Meloni F, et al. Mir-223 is overexpressed in t-lymphocytes of patients affected by rheumatoid arthritis. Hum Immunol. 2010. 71:206–11.

39). Li J., Wan Y., Guo Q., Zou L., Zhang J., Fang Y, et al. Altered microrna expression profile with mir-146a upregulation in cd4+ t cells from patients with rheumatoid arthritis. Arthritis Res Ther. 2010. 12:R81.

40). Murata K., Yoshitomi H., Tanida S., Ishikawa M., Nishitani K., Ito H, et al. Plasma and synovial fluid micrornas as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2010. 12:R86.

41). Nakamachi Y., Kawano S., Takenokuchi M., Nishimura K., Sakai Y., Chin T, et al. Microrna-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2009. 60:1294–304.

42). Nakasa T., Miyaki S., Okubo A., Hashimoto M., Nishida K., Ochi M, et al. Expression of microrna-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008. 58:1284–92.

Table 1.
miRNAs related with rheumatoid arthritis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download