Abstract
Objective
Inflammatory cytokines may play important roles in the pathogenesis of adult onset Still's disease. The enhanced expression of IL-18 was reported in the bone marrow of a Japanese systemic onset juvenile rheumatoid arthritis patient but not in the other organs. To date, there are very few studies relating the bone marrow and AOSD. This study examined the bone marrow findings as well as TNF-α and IL-18 expression in the bone marrow of AOSD patients.
Methods
A retrospective study was performed on 15 AOSD patients who had undergone a bone marrow examination at a university hospital. The clinical and laboratory findings, as well as the bone marrow findings, were analyzed. Immunohistochemistry of IL-18 and TNF-α in bone marrow was performed.
Results
The bone marrow cellularity and myeloid/erythroid cell ratio showed no correlation with the clinical and laboratory findings. TNF-α was expressed at 0.8∼9.8% and IL-18 was expressed at 0.4∼9.8% of bone marrow cells. Cytokine expression was not associated with the clinical patterns of AOSD. The platelet count correlated with the bone marrow TNF-α expression but TNF-α did not correlate with IL-18 expression.
Go to : 
References
2. Cush JJ, Medsger TA Jr, Christy WC, Herbert DC, Cooperstein LA. Adult-onset Still's disease. Clinical course and outcome. Arthritis Rheum. 1987; 30:186–94.
3. Ohta A, Yamaguchi M, Kaneoka H, Nagayoshi T, Hiida M. Adult Still's disease: review of 228 cases from the literature. J Rheumatol. 1987; 14:1139–46.
4. Wouters JM, van de Putte LB. Adult-onset Still's disease; clinical and laboratory features, treatment and progress of 45 cases. Q J Med. 1986; 61:1055–65.
5. Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset still's disease. Ann Rheum Dis. 2006; 65:564–72.

6. Sampalis JS, Medsger Jr TA, Fries JF, Yeadon C, Senecal JL, Myhal D, et al. Risk factors for Adult Still's disease. J Rheumatol. 1996; 23:2049–54.
7. Singh S, Samant R, Joshi VR. Adult onset Still's disease: a study of 14 cases. Clin Rheumatol. 2008; 27:35–9.

8. Mert A, Tabak OF, Bilir M, Ozturk R, Ozdogan H, Aktuglu Y. Fever of unknown origin: a review of 20 patients with Adult-onset Still's disease. Clin Rheumatol. 2003; 22:89–93.

9. Park JH, Bae JH, Choi YS, Lee HS, Jun JB, Jung SS, et al. Adult-onset Still's disease with disseminated intravascular coagulation and multiple organ dysfunctions dramatically treated with cyclosporine A. J Korean Med Sci. 2004; 19:137–41.

10. Pamuk ON, Pamuk GE, Usta U, Cakir N. Hemophagocytic syndrome in one patient with adult-onset Still's disease. Presentation with febrile neutropenia. Clin Rheumatol. 2007; 26:797–800.
11. Arlet JB, Huong DLT, Marinho A, Amoura Z, Wechsler B, Papo T, et al. Reactive haemophagocytic syndrome in adult-onset Still's disease: a report of six patients and a review of the literature. Ann Rheum Dis. 2006; 65:1596–601.

12. Maeno N, Takei S, Imanaka H, Yamamoto K, Kuriwaki K, Kawano Y, et al. Increased interleukin-18 expression in bone marrow of a patient with systemic juvenile idiopathic arthritis and unrecognized macrophage-activation syndrome. Arthritis Rheum. 2004; 50:1935–8.

13. Min JK, Cho CS, Kim HY, Oh EJ. Bone marrow findings in patients with adult Still's disease. Scand J Rheumatol. 2003; 32:119–21.

14. Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol. 1992; 19:424–30.
15. Chen DY, Lan JL, Lin FJ, Hsieh TY, Wen MC. Predominance of Th1 cytokine in peripheral blood and pathological tissues of patients with active untreated adult onset Still's disease. Ann Rheum Dis. 2004; 63:1300–6.

16. Kawashima M, Yamamura M, Taniai M, Yamauchi H, Tanimoto T, Kurimoto M, et al. Levels of Interleukin-18 and its binding inhibitors in the blood circulation of patients with Adult-Onset Still's Disease. Arthritis Rheum. 2001; 44:550–60.

17. Fujii T, Nojima T, Yasuoka H, Satoh S, Nakamura K, Kuwana M, et al. Cytokine and immunogenetic profiles in Japanese patients with adult Still's disease. Association with chronic articular disease. Rheumatology. 2001; 40:1398–404.

18. Choi JH, Suh CH, Lee YM, Suh YJ, Lee SK, Kim SS, et al. Serum cytokine profiles in patients with Adult onset still's disease. J Rheumatol. 2003; 30:24227.
19. Chen DY, Lan JL, Lin FJ, Hsieh TY. Proinflammatory cytokine profiles in sera and pathological tissues of patients with active untreated adult onset Still's disease. J Rheumatol. 2004; 31:2189–98.
20. Kawaguchi Y, Terajima H, Harigai M, Hara M, Kamatani N. Interleukin-18 as a novel diagnostic marker and indicator of disease severity in adult-onset Still's disease. Arthritis Rheum. 2001; 44:1716–8.

21. Hoshino T, Ohta A, Yang D, Kawamoto M, Kikuchi M, Inoue Y, et al. Elevated serum interleukin 6, interferon-γ, and tumor necrosis factor-α levels in patients with adult Still's disease. J Rheumatol. 1998; 25:396–8.
22. Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995; 378:88–91.

23. Asherson RA, Pascoe L, Kraetsch HG, Manger B. Adult onset Still's disease: response to Enbrel. Ann Rheum Dis. 2002; 61:859–60.

24. Kokkinos A, Iliopoulos A, Greka P, Efthymiou A, Katsilambros N, Sfikakis PP. Successful treatment of refractory adult-onset Still's disease with infliximab. A prospective, non-comparative series of four patients. Clin Rheumatol. 2004; 23:45–9.

25. Fautrel B, Sibilia J, Mariette X, Combe B, the Club Rhumatismes et inflammation. Tumour necrosis factor α blocking agents in refractory adult Still's disease: an observational study of 20 cases. Ann Rheum Dis. 2005; 64:262–6.
26. Park IH, Park MC, Choi ST, Park YB, Lee SK. Refractory cases of adult onset Still's disease successfully treated with TNF-α blocker. J Korean Rheum Assoc. 2005; 12:335–40.
27. Woo H, Kim OK, Lee GH, Park JJ, Lee SS, Park YW. Successful treatment with etanercept in a patient with adult-onset Still's diseases. J Korean Rheum Assoc. 2006; 13:306–10.
Go to : 
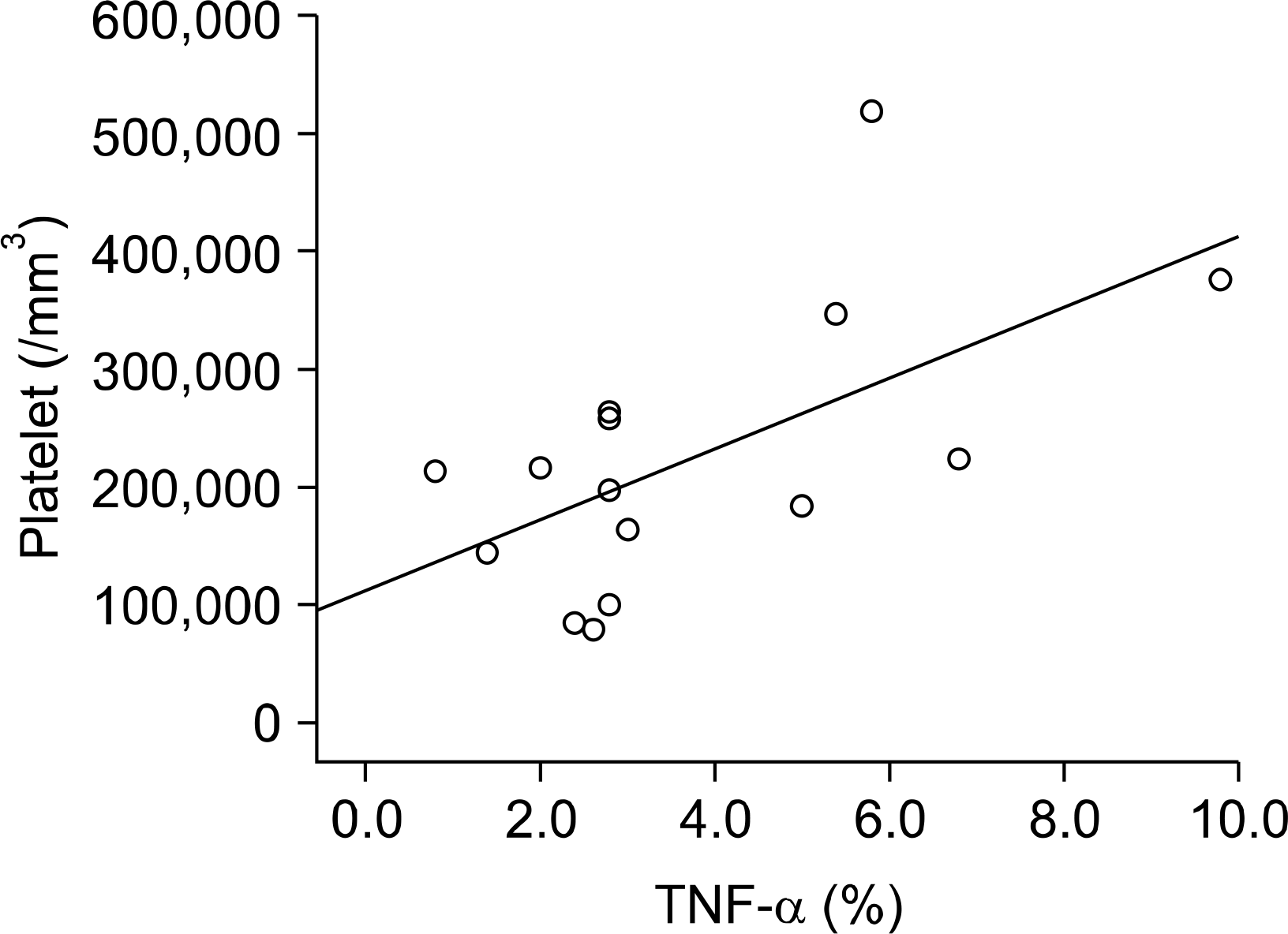 | Fig. 1.Correlation between bone marrow TNF-α expression and platelet counts (The correlation coefficient was analyzed by Sperman's rank correlation test. n=15, r=0.578, p<0.005). |
Table 1.
Clinical characteristics of AOSD patients
Table 2.
Laboratory findings of the AOSD patients
Table 3.
TNF-α and IL-18 expression in the bone marrow of AOSD patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download