Abstract
Objective
Alterations in the immune system occur with aging, and these contribute to an increased risk of infection and malignancy. The age-associated changes in T cell immunity range from single cell function to the maintenance of cell populations. We investigated the kinetics of CD4+ T cell activation and proliferation in young and elderly subjects after stimulating their peripheral blood mononuclear cells with anti-CD3 and anti-CD28 antibodies (Abs).
Methods
The expressions of the activation markers CD69, CD40L and CD25 on the CD4+ T cells from young (n=14) and elderly (n=19) were analyzed at 6, 24 and 48 hours (hrs) of T cell receptor (TCR) stimulation by using flow cytometry. In the same individuals, the CD4+ T cell proliferation was determined at 48 and 96 hrs of TCR stimulation by using the CFSE dilution method.
Results
The elderly had decreased CD69 and CD40L expressions on the CD4+ T cells at 6 hrs of stimulation, as compared to that of the young patients. The elderly also had a decreased CD25 expression on the CD4+ T cells at 24 hrs of stimulation. However, the two groups had similar levels of the CD25, CD69 and CD40L expressions at 48 hrs of stimulation. The elderly had decreased CD4+ T cell proliferation at 96 hrs of stimulation, as compared to that of the young, although both groups had similar levels of CD4+ T cell proliferation at 48 hrs of stimulation.
Go to : 
References
1. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004; 5:133–9.

2. Miller RA. Aging and Immune Function. In: Paul WE, ed. Fundamental Immunology. 4th ed. p.947–966. Philadelphia, Lippincott-Raven,. 1999.
3. Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005; 174:7446–52.

4. Nikolich-Zugich J. T cell aging: naive but not young. J Exp Med. 2005; 201:837–40.
6. Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000; 31:578–85.

7. Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000; 18:529–60.

8. Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005; 17:468–75.

9. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002; 2:251–62.

13. Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008; 127:107–18.

14. Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004; 173:673–81.
15. Ginn-Pease ME, Whisler RL. Alterations in the expression of interleukin-2R subunits by activated T cells from elderly humans are uncoupled from aberrancies in G1/S progression. J Interferon Cytokine Res. 2001; 21:515–21.

16. Pawelec G, Adibzadeh M, Solana R, Beckman I. The T cell in the ageing individual. Mech Ageing Dev. 1997; 93:35–45.
17. Schindowski K, Frohlich L, Maurer K, Muller WE, Eckert A. Age-related impairment of human T lym-phocytes' activation: specific differences between CD4 (+) and CD8 (+) subsets. Mech Ageing Dev. 2002; 123:375–90.
18. Fernandez-Gutierrez B, Jover JA, De Miguel S, Hernandez-Garcia C, Vidan MT, Ribera JM, et al. Early lymphocyte activation in elderly humans: impaired T and T-dependent B cell responses. Exp Gerontol. 1999; 34:217–29.
19. Beckman I, Dimopoulos K, Xu XN, Bradley J, Henschke P, Ahern M. T cell activation in the elderly: evidence for specific deficiencies in T cell/accessory cell interactions. Mech Ageing Dev. 1990; 51:265–76.

20. Douziech N, Seres I, Larbi A, Szikszay E, Roy PM, Arcand M, et al. Modulation of human lymphocyte proliferative response with aging. Exp Gerontol. 2002; 37:369–87.

21. Tortorella C, Pisconti A, Piazzolla G, Antonaci S. APC-dependent impairment of T cell proliferation in aging: role of CD28- and IL-12/IL-15-mediated signaling. Mech Ageing Dev. 2002; 123:1389–402.

22. Sansoni P, Fagnoni F, Vescovini R, Mazzola M, Brianti V, Bologna G, et al. T lymphocyte proliferative capability to defined stimuli and costimulatory CD28 pathway is not impaired in healthy centenarians. Mech Ageing Dev. 1997; 96:127–36.

23. Glimm H, Eaves CJ. Direct evidence for multiple self-renewal divisions of human in vivo repopulating hematopoietic cells in short-term culture. Blood. 1999; 94:2161–8.

24. Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994; 12:881–922.

27. Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005; 11:1118–24.

28. Kahi S, Cozon GJ, Greenland T, Wallon M, Gay-Andrieu F, Peyron F. A rapid flow cytometric method to explore cellular immunity against Toxoplasma gondii in humans. Clin Diagn Lab Immunol. 1998; 5:745–8.
29. Maino VC, Suni MA, Ruitenberg JJ. Rapid flow cytometric method for measuring lymphocyte subset activation. Cytometry. 1995; 20:127–33.

30. Michalek J, Collins RH, Durrani HP, Vaclavkova P, Ruff LE, Douek DC, et al. Definitive separation of graft-versus-leukemia- and graft-versus-host-specific CD4+ T cells by virtue of their receptor beta loci sequences. Proc Natl Acad Sci USA. 2003; 100:1180–4.
31. Eisenbraun MD, Tamir A, Miller RA. Altered composition of the immunological synapse in an anergic, age-dependent memory T cell subset. J Immunol. 2000; 164:6105–12.

32. Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001; 166:3151–7.

33. Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000; 165:1243–51.

34. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999; 401:708–12.

35. Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994; 55:876–82.
36. Weng NP, Hathcock KS, Hodes RJ. Regulation of telomere length and telomerase in T and B cells: a mechanism for maintaining replicative potential. Immunity. 1998; 9:151–7.
Go to : 
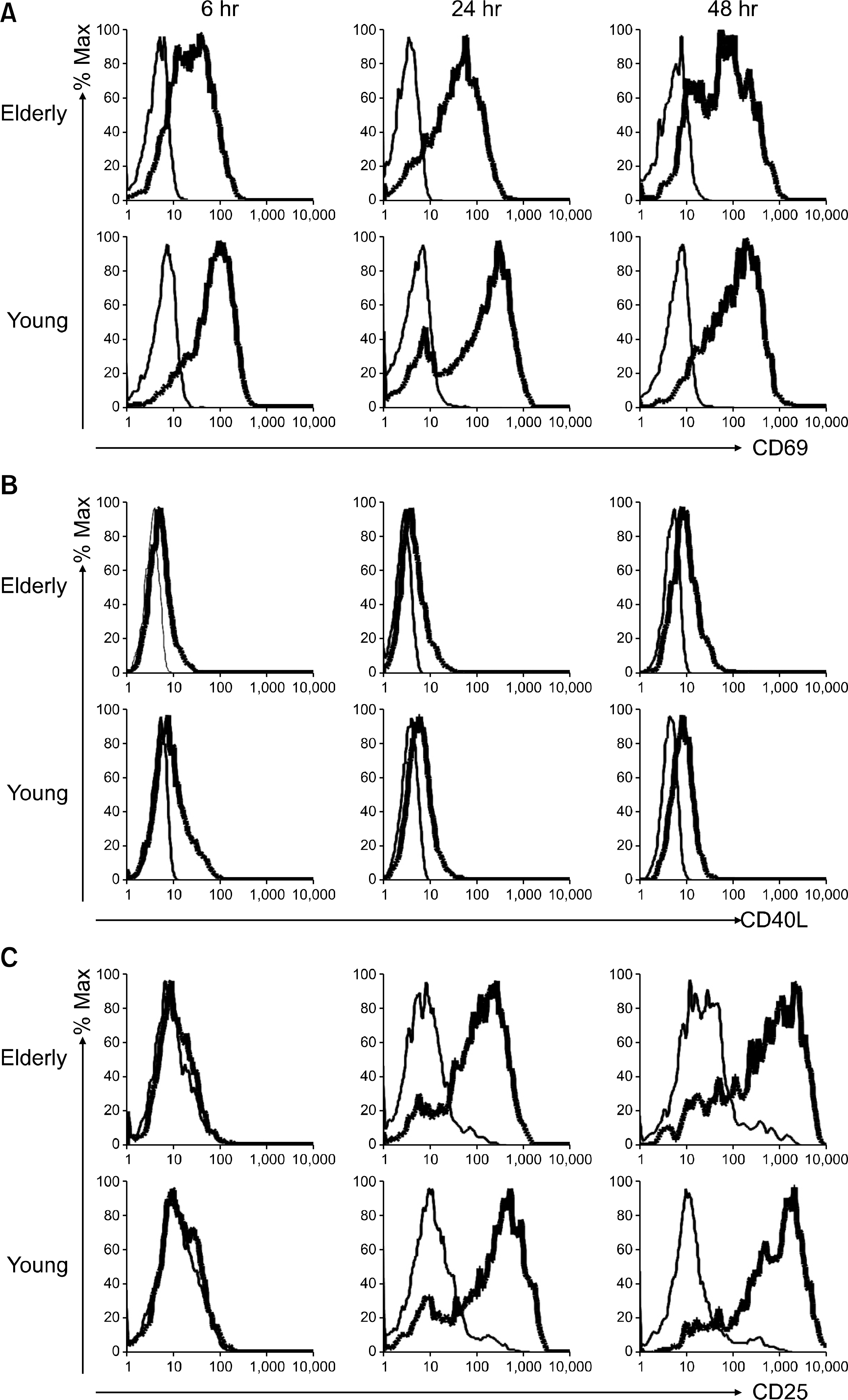 | Fig. 1.The expressions of CD 69, CD40L and CD25 by the CD4+ T cells in response to anti-CD3 and -CD28 antibody (Ab) stimulation. Peripheral blood mononuclear cells from a young individual and an elderly individual were stimulated for 6, 24 and 48 hours (hr) with PBS (thin line) or anti-CD3 Abs (bold line) at 10 μg/mL in the presence of anti-CD28 Abs (1 μg/ mL). The stimulated cells were stained with anti-CD4, -CD69 (A), -CD 40L (B) or -CD25 (C) Abs. The cells were washed and then analyzed on a flow cytometer. The cells were gated on the CD4+ population. |
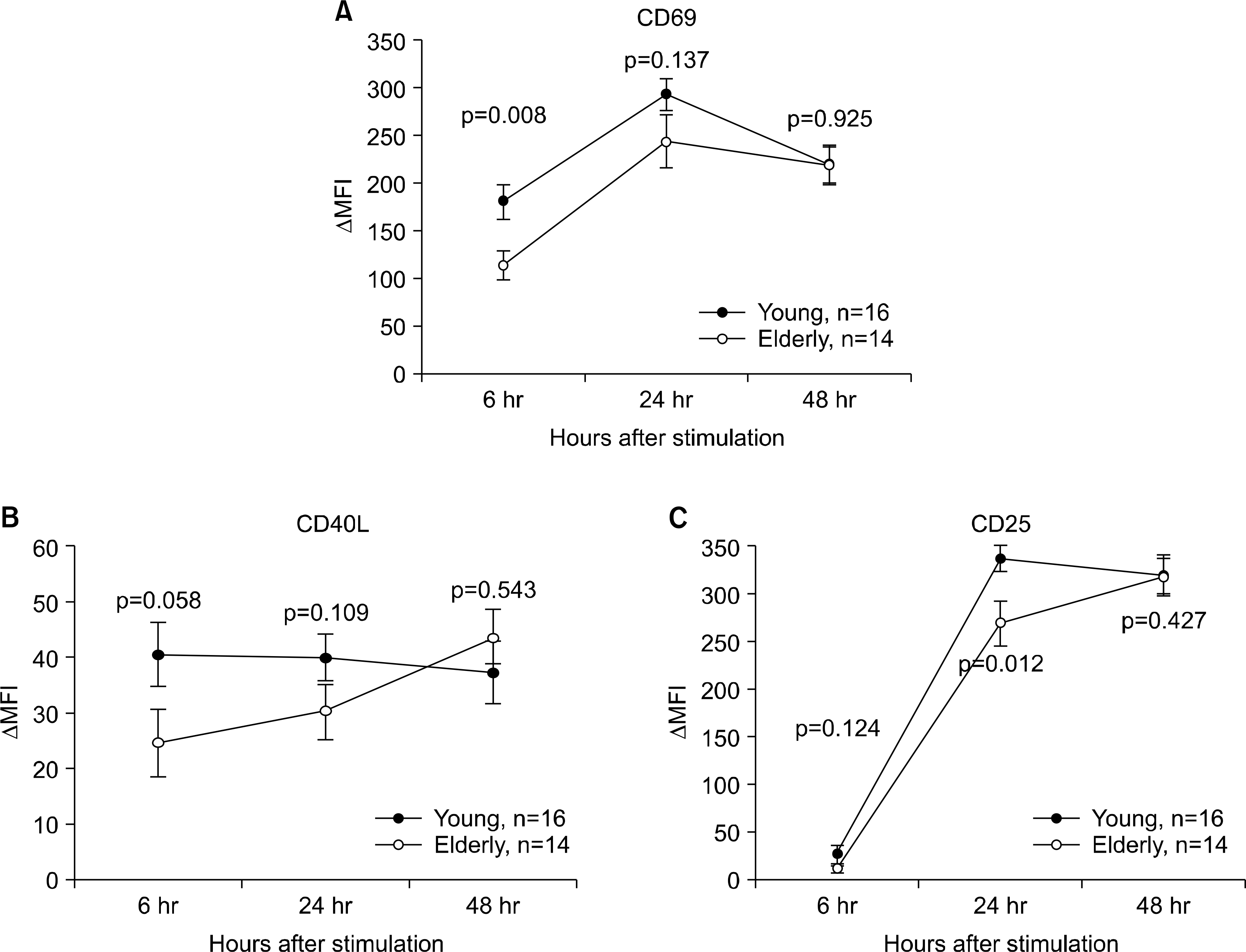 | Fig. 2.Kinetics of the activation markers' expressions on the CD4+ T cells in young and elderly people. Peripheral blood mononuclear cells from 14 young people and 19 elderly people were stimulated for 6, 24 and 48 hours (hr) with PBS or anti-CD3 Abs at 10 μg/mL in the presence of anti-CD28 Abs (1 μg/mL). The stimulated cells were stained with anti-CD4, -CD69 (A), -CD40L (B) or -CD25 (C) Abs. The cells were washed and analyzed on a flow cytometer. The cells were gated on the CD4+ population. The differences in median fluorescent intensity (ΔMFI) between the samples stimulated with PBS and anti-CD3 Abs were obtained. The symbols and the error bars indicate the mean ΔMFI and the S.E.M, respectively. The values on the Y-axis indicate the ΔMFI. The p-values were obtained by the Mann-Whitney U test. |
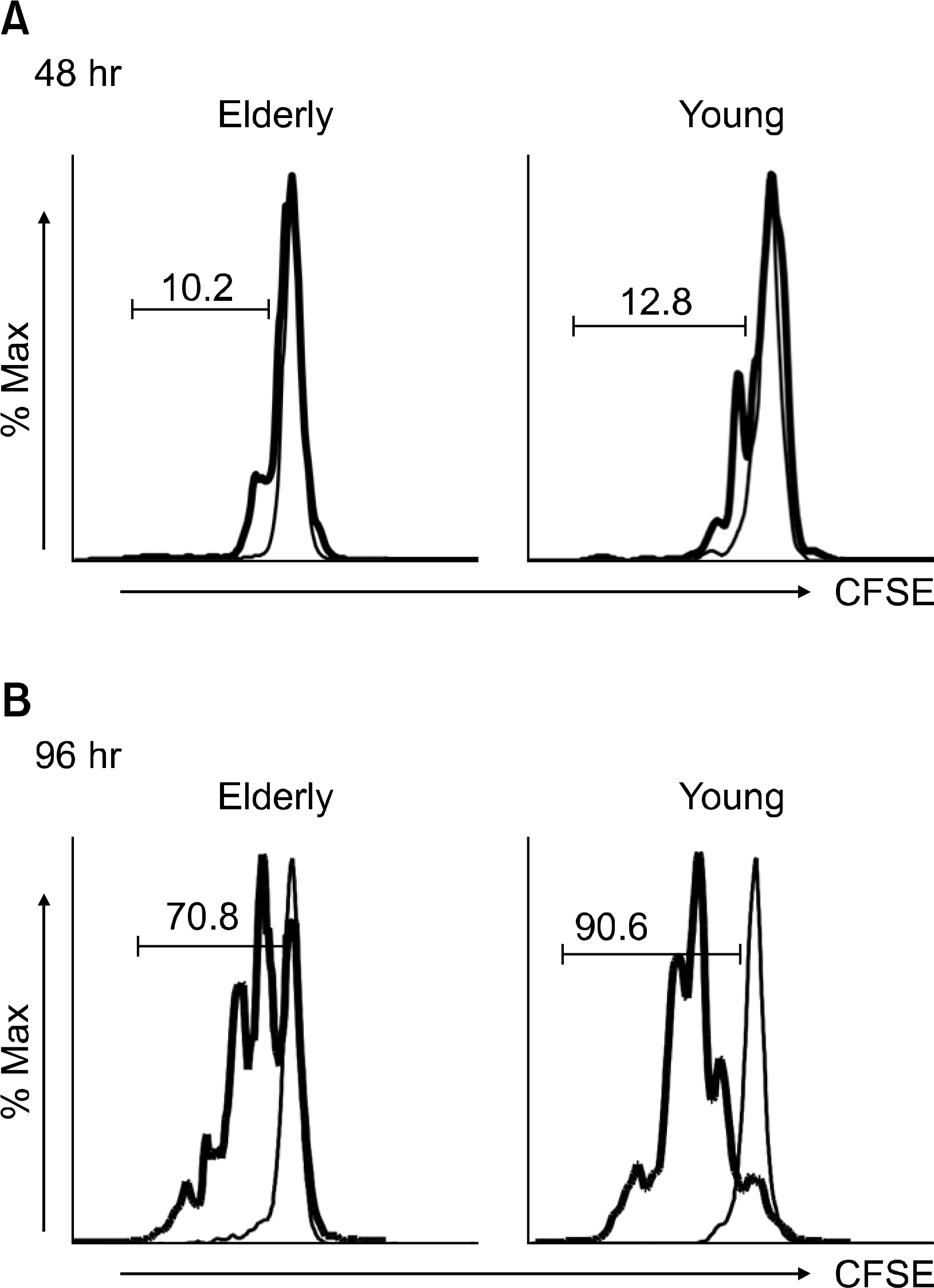 | Fig. 3.The proliferation of CD4+ T cells in response to anti-CD3 and -CD28 antibody (Ab) stimulation as measured by carboxyfluorescein succinimidyle ester (CFSE) staining. The peripheral blood mononuclear cells from a young individual and an elderly individual were stained with CFSE. The cells were then stimulated for 48 (A) and 96 (B) hours (hr) with PBS (thin line) or anti-CD3 Abs (bold line) at 10 μg/mL in the presence of anti-CD28 Abs (1 μg/mL). The stimulated cells were stained with anti-CD4 Abs, washed and analyzed on a flow cytometer. The cells were gated on the CD4+ population. The numbers on the histograms indicate the percentage of proliferated cells in the samples stimulated with anti-CD3 Abs. |
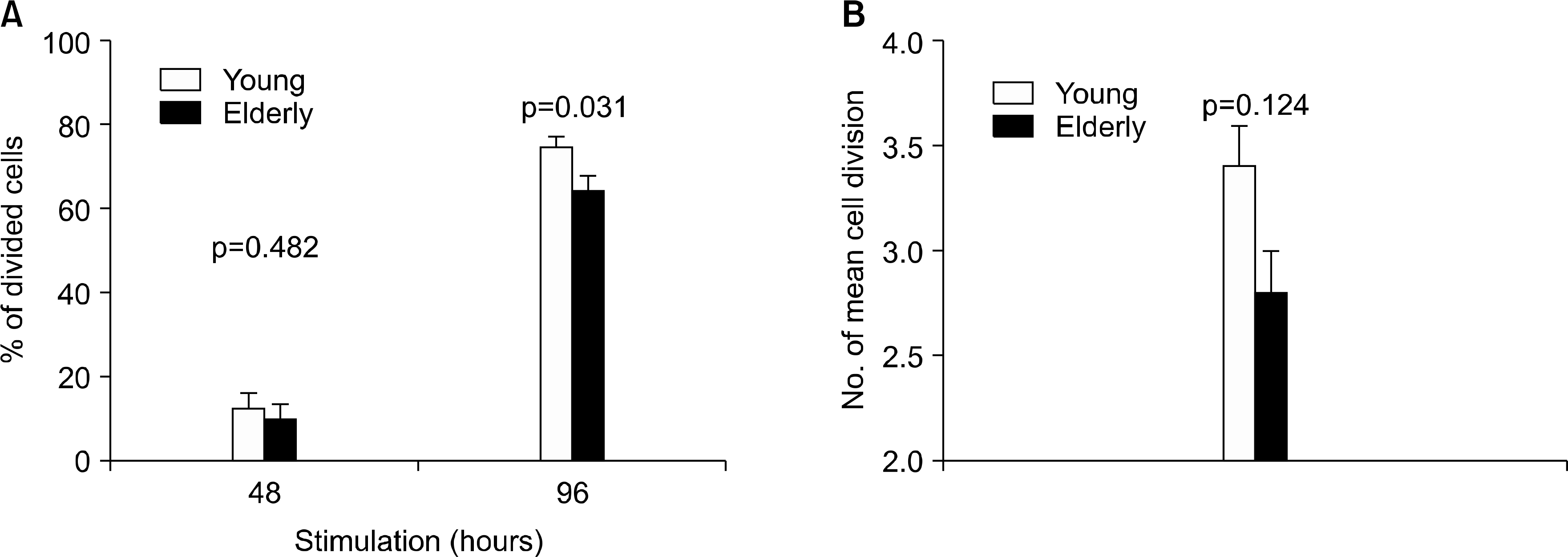 | Fig. 4.Difference in the proliferation of CD4+ T cells in response to anti-CD3 and -CD28 Ab stimulation between the young and the elderly. Peripheral blood mononuclear cells (PBMCs) from young and elderly people were stained with CFSE. The cells were then stimulated for 48 or 96 hours (hr) with PBS or anti-CD3 Abs at 10 μg/mL in the presence of anti-CD28 Abs (1 μg/mL). The stimulated cells were stained with anti-CD4 Abs. The cells were washed and analyzed on a flow cytometer. The data for 48 hr were from 7 young and 7 elderly individuals (A). The data for 96 hr were from 14 young and 16 elderly individuals (A and B). The columns and the error bars indicate the mean percent of proliferating CD4+ T cells and the S.E.M (A) or the number of mean divisions and S.E.M (B). The p-values were obtained by the Mann-Whitney U test. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download