Abstract
Objective
Bucillamine is a disease-modifying antirheumatic drug that's widely used in Korea and Japan, and it is reported to be a cause of proteinuria. However, the clinical course of the nephropathy associated with the use of bucillamine in rheumatoid arthritis patients has not been described in detail in Korea.
Methods
We examined clinical records of 835 patients who were treated with bucillamine for rheumatoid arthritis at least 2 months at Ajou University Hospital from 2003 to 2008, and we found 23 patients (2.75%) with proteinuria. Each patient was followed up until the proteinuria had resolved.
Results
At the time the proteinuria developed, the mean age of patients was 53.8±11.0 years. Only one patient had marked hypoalbuminemia (<3.0 g/dL). The mean value of the random urine protein-creatinine ratio was 3.44±2.99. The proteinuria appeared 4∼18 months after the initiation of the treatment with bucillamine. Among the patients, renal biopsy was carried out in 18 patients, and pathological findings were 17 cases of membranous glomerulopathy and 1 case of focal segmental glomerulosclerosis. On the follow-up of the 18 patients, the proteinuria in all the patients had resolved completely without deterioration of renal function. But the time to resolution of the proteinuria was positively correlated with the length of bucillamine treatment after the onset of proteinuria (p<0.001, r=0.744).
References
2. Dische FE, Swinson DR, Hamilton EB, Parsons V. Immunopathology of penicillamine-induced glomerular disease. J Rheumatol. 1984; 11:584–5.
3. Golding JR, Andrews FM, Camp V, Day AT, Freeman AM, Golding DN, et al. Controlled trial of penicillamine in severe rheumatoid arthritis. Ann Rheum Dis. 1973; 32:385–6.

5. Hall CL, Fothergill NJ, Blackwell MM, Harrison PR, MacKenzie JC, MacIver AG. The natural course of gold nephropathy: long term study of 21 patients. Br Med J (Clin Res Ed). 1987; 295:745–8.

6. Hall CL, Jawad S, Harrison PR, MacKenzie JC, Bacon PA, Klouda PT, et al. Natural course of penicillamine nephropathy: a long term study of 33 patients. Br Med J (Clin Res Ed). 1988; 296:1083–6.

7. Goto M, Sasano M, Nishioka K. Decrease in disease activity and concomitant increase in the percentage of peripheral blood suppressor T-cells in rheumatoid arthritis by a newly synthesized slow-acting antirheumatic drug (Bucillamine). Int J Immunopharmacol. 1989; 11:327–31.

8. Nagahama K, Matsushita H, Hara M, Ubara Y, Hara S, Yamada A. Bucillamine induces membranous glomerulonephritis. Am J Kidney Dis. 2002; 39:706–12.

9. Obayashi M, Uzu T, Harada T, Yamato M, Takahara K, Yamauchi A. Clinical course of bucillamineinduced nephropathy in patients with rheumatoid arthritis. Clin Exp Nephrol. 2003; 7:275–8.

10. Hoshino J, Ubara Y, Hara S, Suwabe T, Sawa N, Tagami T, et al. Outcome and treatment of bucillamine-induced nephropathy. Nephron Clin Pract. 2006; 104:15–9.

11. Bae SC, Lee IH, Yoo DH, Kim SY. Clinical trial of N-(2-Mercapto-2-methylpropionyl)-L-cystein (SA96) in patients with rheumatoid arthritis. Korean J Med. 1993; 44:416–24.
12. Song CH, Lee JS, Lee CH, Lee CW, Suh CH, Song JS, et al. The effect of bucillamine in the inital treatment of rheumatoid arthritis and treatment of patients with refractory rheumatoid arthritis. J Korean Rheum Assoc. 1998; 5:83–8.
13. Helin H, Korpela M, Mustonen J, Pasternack A. Mild mesangial glomerulopathy–a frequent finding in rheumatoid arthritis patients with hematuria or proteinuria. Nephron. 1986; 42:224–30.
14. Helin HJ, Korpela MM, Mustonen JT, Pasternack AI. Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum. 1995; 38:242–7.

15. Salomon MI, Gallo G, Poon TP, Goldblat MV, Tchertkoff V. The kidney in rheumatoid arthritis. A study based on renal biopsies. Nephron. 1974; 12:297–310.
16. Koseki Y, Terai C, Moriguchi M, Uesato M, Kamatani N. A prospective study of renal disease in patients with early rheumatoid arthritis. Ann Rheum Dis. 2001; 60:327–31.

17. Nakano M, Ueno M, Nishi S, Shimada H, Hasegawa H, Watanabe T, et al. Analysis of renal pathology and drug history in 158 Japanese patients with rheumatoid arthritis. Clin Nephrol. 1998; 50:154–60.
Fig. 1.
Correlation between interval to remission of proteinuria and bucillamine exposure time after onset of proteinuria.
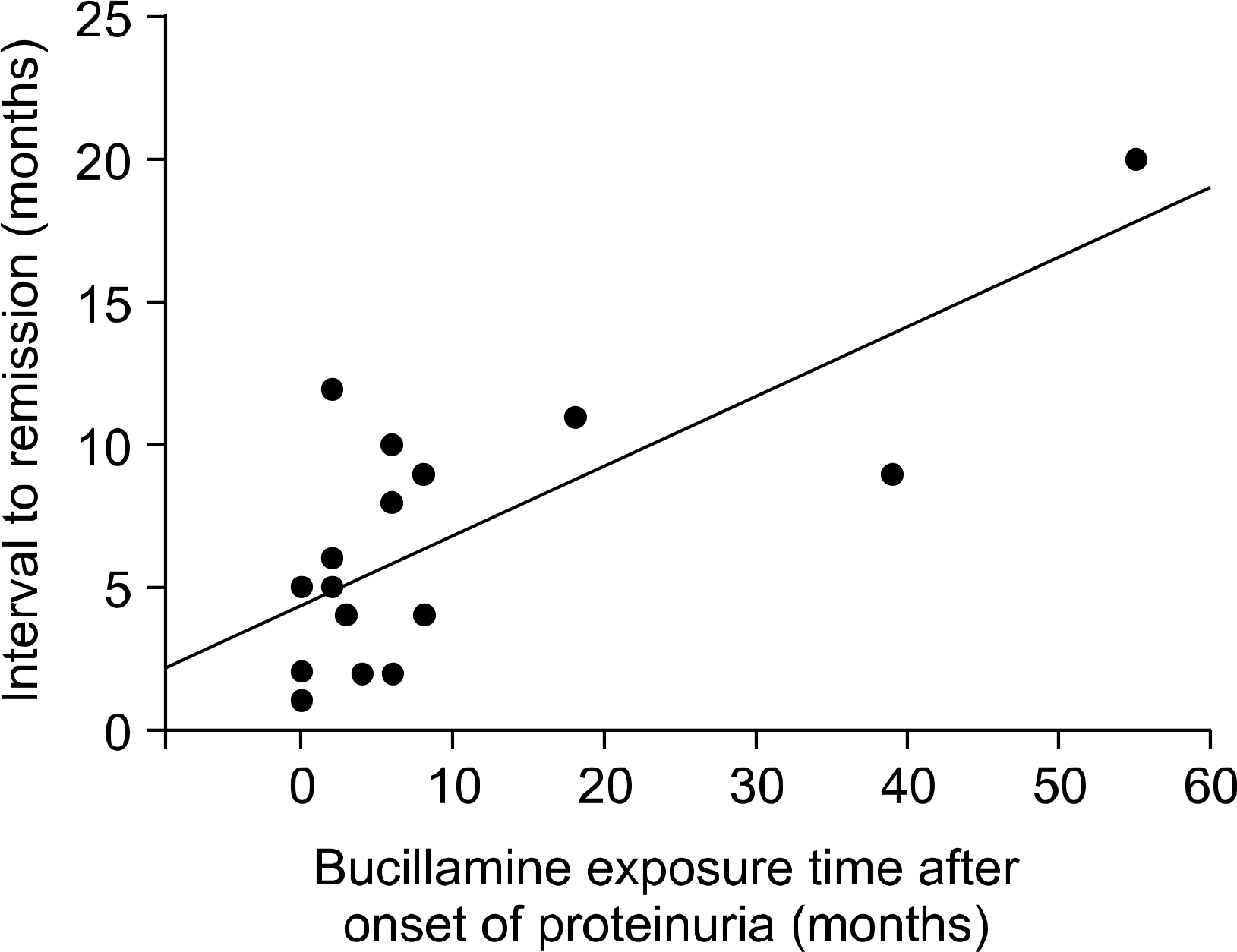
Table 1.
Clinical characteristics of patients
Table 2.
Bucillamine treatment and proteinuria
| Mean±SD | |
|---|---|
| Length of bucillamine treatment (months) | 7.39±3.72 |
| Dose of bucillamine (mg/day) | 200 |
| Length of bucillaminetreatment after onset of proteinuria (months) | 8.25±3.16 (3.12±1.83∗) |
| Time to resolution of proteinuria (months) | 6.5±4.84 (5.13±3.4∗) |
Table 3.
Pathologic findings in renal biopsy specimens of patients with bucillamine-induced nephropathy
Table 4.
Factors influencing interval to remission
Table 5.
Relationship between clinical parameters and the interval to remission




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download