Abstract
Objective
Leflunomide is the newest disease-modifying anti-rheumatic drug (DMARD) that is known to have an equivalent clinical efficacy and tolerability to methotrexate (MTX). Previous studies reported that a co-treatment with MTX and leflunomide can induce additive clinical improvements in RA patients. However, a previous study also demonstrated a reversible elevation of the transaminase levels in up to 63% of patients administered a combination treatment of leflunomide and MTX. This study examined the hepatotoxicity of a combination treatment of MTX and leflunomide.
Methods
From March, 2004, to February, 2006, 203 patients who had been treated in 3 rheumatology clinics, Goyang city, South Korea, were reviewed retrospectively. The data showed that 38.92% of patients scored higher than grade 1 hepatotoxicity and 6.90% of patients scored higher than grade 2 according to the NCI/NIH (National Cancer Institute/National Institutes of Health) Common Toxicity Criteria.
Results
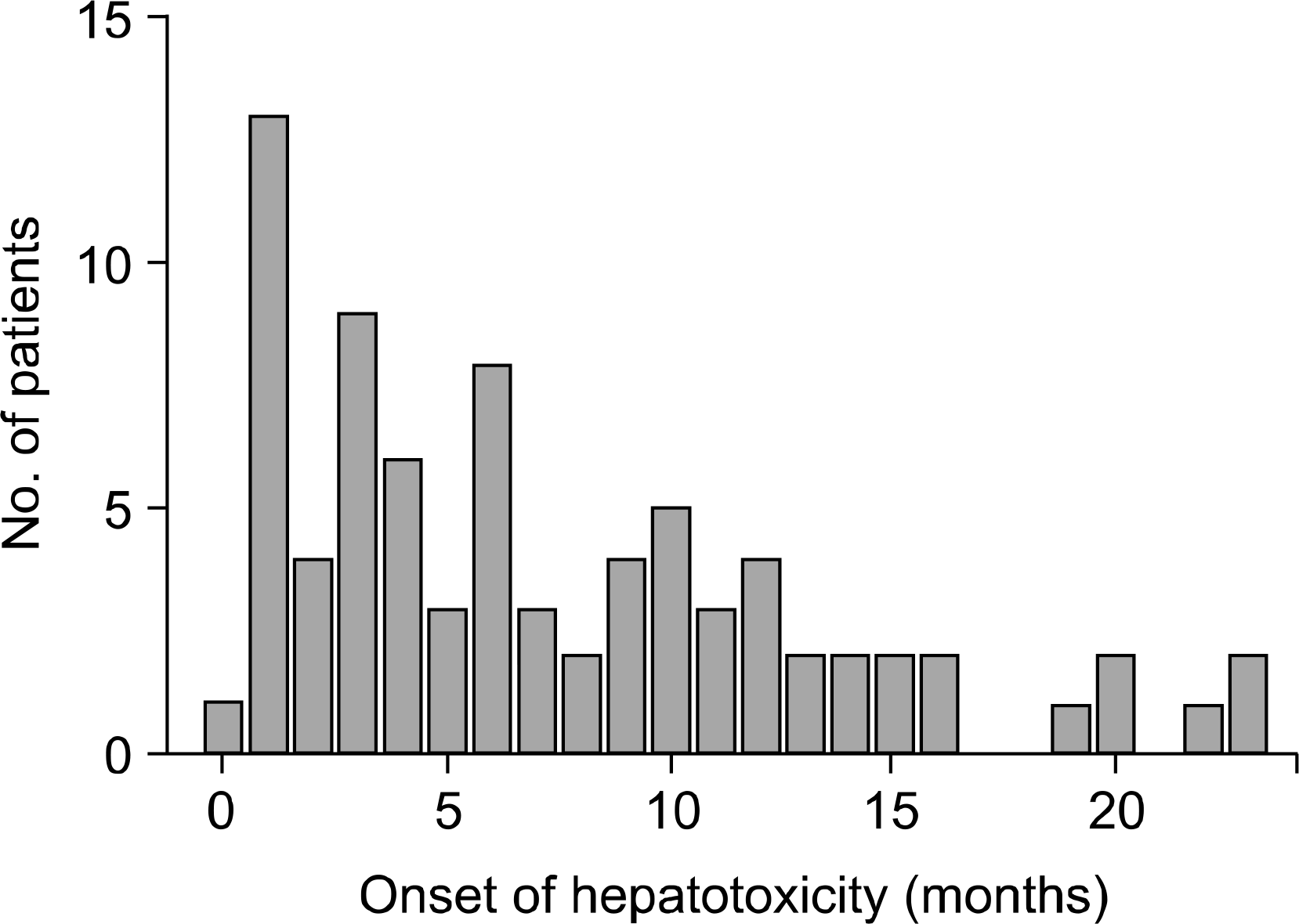
The median onset time of hepatotoxicity was 5.91 months after treatment. Leflunomide administration was stopped in 39 patients due to several adverse reactions. Among the 39 patients, hepatotoxicity was observed in only 20.51%, suggesting that the hepatotoxicity was not more frequent than expected. Hepatotoxicity did not increase in proportion to the dose of leflunomide and MTX, age, gender, and disease activity.
Go to : 
References
1. Fox RI. Mechanism of action of leflunomide in rheumatoid arthritis. J Rheumatol. 1998; 53:20–6.
2. Li EK, Tam LS, Tomlinson B. Leflunomide in the treatment of rheumatoid arthritis. Clin Ther. 2004; 26:447–59.

3. Dale J, Alcorn N, Capell H, Madhok R. Combination therapy for rheumatoid arthritis: methotrexate and sulfasalazine together or with other DMARDs. Nat Clin Pract Rheumatol. 2007; 3:450–8.

4. Weinblatt ME, Dixon JA, Falchuk KR. Serious liver disease in a patient receiving methotrexate and leflunomide. Arthritis Rheum. 2000; 43:2609–13.

5. Kremer JM, Genovese MC, Cannon GW, Caldwell JR, Cush JJ, Furst DE, et al. Concomitant leflunomide therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate. A randomized, double-blind, placebocontrolled trial. Ann Intern Med. 2002; 137:726–33.
6. Kremer J, Genovese M, Cannon GW, Caldwell J, Cush J, Furst DE, et al. Combination leflunomide and methotrexate (MTX) therapy for patients with active rheumatoid arthritis failing MTX monotherapy: open-label extension of a randomized, double-blind, placebo controlled trial. J Rheumatol. 2004; 31:1521–31.
7. American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis 2002 Update. Arthritis Rheum. 2002; 46:328–46.
8. van Roon EN, Jansen TL, Houtman NM, Spoelstra P, Brouwers JR. Leflunomide for the treatment of rheumatoid arthritis in clinical practice: incidence and severity of hepatotoxicity. Drug Saf. 2004; 27:345–52.
9. Sevilla-Mantilla C, Ortega L, Agúndez JA, Fer-nández-Gutiérrez B, Ladero JM, Díaz-Rubio M. Leflunomide-induced acute hepatitis. Dig Liver Dis. 2004; 36:82–4.

10. Martin N, Innes JA, Lambert CM, Turnbull CM, Wallace WA. Hypersensitivity pneumonitis associated with leflunomide therapy. J Rheumatol. 2007; 34:1934–7.
11. Marzano AV, Ramoni S, Del Papa N, Barbareschi M, Alessi E. Leflunomide-induced subacute cutaneous lupus erythematosus with erythema multiforme-like lesions. Lupus. 2008; 17:329–31.
12. Toyokawa Y, Kingetsu I, Yasuda C, Yasuda J, Yoshida K, Kurosaka D, et al. Pancytopenia, including macrocytic anemia, associated with leflunomide in a rheumatoid arthritis patient. Mod Rheumatol. 2007; 17:436–40.

13. Chambers C, Koren G, Tutuncu ZN, Johnson D, Jones KL. Are new agents used to treat rheumatoid arthritis safe to take during pregnancy? Organization of Teratology Information Specialists (OTIS) study. Can Fam Physician. 2007; 53:409–12.
14. Gupta R, Gupta SK. Severe hepatotoxicity in a rheumatoid arthritis patient switched from leflunomide to methotrexate. Med Gen Med. 2005; 7:9.
15. Shergy WJ, Polisson RP, Caldwell DS, Rice JR, Pisetsky DS, Allen NB. Methotrexate associated hepatotoxicity: retrospective analysis of 210 patients with rheumatoid arthritis. Am J Med. 1988; 85:771–4.
16. Mladenovic V, Domljan Z, Rozman B, Jajic I, Mihajlovic D, Dordevic J, et al. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis: results of a randomized, placebocontrolled, phase II study. Arthritis Rheum. 1995; 38:1595–603.
17. Kalden JR, Schattenkirchner M, Sorensen H, Emery P, Deighton C, Rozman B, et al. The efficacy and safety of leflunomide in patients with active rheumatoid arthritis: a five-year follow up study. Arthritis Rheum. 2003; 48:1513–20.
Go to : 
Table 1.
NCI/NIH∗ Common Toxicity Criteria
| 0 | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| AST/ALT | WNL† | >ULN‡−2.5×ULN | >2.5−5.0×ULN | >5.0−20.0×ULN | >20.0×ULN |
| Albumin | WNL | <LLN§−3 g/dL | 2−<3 g/dL | <2 g/dL | − |
| Bilirubin | WNL | >ULN−1.5×ULN | >1.5−3.0×ULN | >3.0−10.0×ULN | >10.0×ULN |
Table 2.
The characteristics of the patient groups
| Hepatotoxicity (+) n=124 | Hepatotoxicity (−) n=79 | p-value | |
|---|---|---|---|
| Age (year) | 51.6±11.7 | 51.6±13.2 | 0.998 |
| Sex [male (%)] | 10.1 | 14.5 | 0.362 |
| Disease duration (month) | 66.5±71.0 | 50.7±61.4 | 0.094 |
| ESR (mm/hr) | 29.0±26.6 | 30.2±25.4 | 0.748 |
| CRP (mg/dL) | 2.0±4.4 | 2.2±6.7 | 0.799 |
| TJC∗ | 12.9±12.6 | 13.2±12.9 | 0.905 |
| SJC† | 12.4±12.1 | 12.7±13.0 | 0.877 |
| RF positive [n (%)]‡ | 34 (45.3) | 63 (52.9) | 0.302 |
| Anti-CCP positive [n (%)]§ | 23 (46.0) | 35 (44.9) | 0.900 |
| Previous use of MTX [n (%)] | 49 (39.5) | 62 (78.5) | 0.105 |
| Follow-up duration (month) | 14.2±6.0 | 16.4±6.8 | 0.018 |
Table 3.
Incidence of hepatotoxicity according to the NCI/NIH Common Toxicity Criteria
| Number | % | |
|---|---|---|
| Grade 1 | 65 | 32.0 |
| Grade 2 | 13 | 6.4 |
| Grade 3 | 1 | 0.5 |
| Grade 4 | 0 | 0 |
| Total | 79 | 38.9 |
Table 4.
Comparisons in according to the dosage of leflunomide and MTX




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download