Abstract
Objective
Indoleamine 2, 3-dioxygenase (IDO), an immuno suppression enzyme, is one of the initial and rate-limiting enzymes involved in the catabolism of the essential amino acid tryptophan. IDO inhibits T cell proliferation, induces T cell apoptosis, and plays a fundamental role in autoimmunity and allergy. We investigated which subtype of dendritic cells (DCs) is involved in IDO expression and the generation of regulatory T cells during the induction of oral tolerance in type II collagen-induced arthritis (CIA).
Methods
Type II Collagen was fed to DBA/1J mice before immunization. Changes in DC subtypes and induction of regulatory T cell in orally tolerized CIA mice were analyzed. Whether the effect of DC subtype was modulated by the IDO expression, was determined by flow cytometry (FACs) and confocal microscopy.
Results
IDO expression of CD11c+ DCs was higher in orally tolerized CIA mice than in non-tolerized CIA mice. CD11b+ DCs of the CD11c+DCs, subtype was higher in the induction of in IDO expression. Our data suggest that these IDO expressing DCs of oral tolerized mice suppressed type II collagen-specific T cell proliferation and favored the differentiation of naïve CD4+ T cells into regulatory T cells. Especially, CD11c+CD11b+ DCs expressed IDO, which is known to be associated with regulatory T cell induction.
Go to : 
References
1. Nagler-Anderson C, Bober LA, Robinson ME, Siskind GW, Thorbecke GJ. Suppression of type II collageninduced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci U S A. 1986; 83:7443–6.

2. Garcia G, Komagata Y, Slavin AJ, Maron R, Weiner HL. Suppression of collageninduced arthritis by oral or nasal administration of type II collagen. J Autoimmun. 1999; 13:315–24.

3. Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci U S A. 1994; 91:6688–92.

4. Mellor AL, Munn D, Chandler P, Keskin D, Johnson T, Marshall B, et al. Tryptophan catabolism and T cell responses. Adv Exp Med Biol. 2003; 527:27–35.

5. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004; 4:762–4.

6. Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002; 1:609–20.

7. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb J. 1991; 5:2516–22.
8. Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002; 99:351–8.

9. Derry CJ, Harper N, Davies DH, Murphy JJ, Staines NA. Importance of dose of type II collagen in suppression of collageninduced arthritis by nasal tolerance. Arthritis Rheum. 2001; 44:1917–27.

10. Daniels LK: Rapid in-office and in-vivo desensitization of an injection phobia utilizing hypnosis. Am J Clin Hypn. 1976; 18:200–3.
11. Mowat AM, Parker LA, Beacock-Sharp H, Millington OR, Chirdo F. Oral tolerance: overview and historical perspectives. Ann N Y Acad Sci. 2004; 1029:1–8.

12. Dubois B, Goubier A, Joubert G, Kaiserlian D. Oral tolerance and regulation of mucosal immunity. Cell Mol Life Sci. 2005; 62:1322–32.
13. Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol Rev. 2005; 206:132–48.

14. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002; 297:1867–70.

15. Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003; 24:242–8.

16. Min SY, Park KS, Cho ML, Kang JW, Cho YG, Hwang SY, et al. Antigen-induced, tolerogenic CD11c+, CD11b+ dendritic cells are abundant in Peyer's patches during the induction of oral tolerance to type II collagen and suppress experimental collageninduced arthritis. Arthritis Rheum. 2006; 54:887–98.
17. Grohmann U, Fallarino F, Silla S, Bianchi R, Belladonna ML, Vacca C, et al. CD40 ligation ablates the tolerogenic potential of lymphoid dendritic cells. J Immunol. 2006; 166:277.

18. Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenasedependent T cell regulatory functions via IFN type 1 signaling. J Immunol. 2005; 175:5601–5.

19. Fallarino F, Gizzi S, Mosci P, Grohmann U, Puccetti P. Tryptophan catabolism in IDO+ plasmacytoid dendritic cells. Curr Drug Metab. 2007; 8:209–16.

20. Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebocontrolled trial. Arthritis Rheum. 1998; 41:290–7.

21. Park MJ, Min SY, Park KS, Cho YG, Cho ML, Jung YO, et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells are involved in the generation of CD4+CD25+ regulatory T cells in Peyer's patches in an orally tolerized, collageninduced arthritis mouse model. Arthritis Res Ther. 2008; 10:11R.

22. Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999; 20:469–73.

23. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999; 189:1363–72.

24. Bozza S, Fallarino F, Pitzurra L, Zelante T, Montagnoli C, Bellocchio S, et al. A crucial role for tryptophan catabolism at the host/Candida albicans interface. J Immunol. 2005; 174:2910–8.
25. Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β.interleukin 4, and prosta-glandinnE expression in the brain. J Exp Med. 1992; 176:1355–64.
26. Weiner HL, Mackin GA, Matusi M, Orav EJ, Khoury SJ, Dawson DM, et al. Double blind pilot trial of oral tolerance with myelin antigen in multiple sclerosis. Science. 1993; 259:1321–4.
27. Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A. 1991; 88:10252–6.

28. Nussenblatt RB, Capsi R, Mahdi R, Cahn CC, Roberge F, Lider O, et al. Inhibition of S-antigen induced experimental autoimmune uveoretinitis by oral inducton of tolerance with S-antigen. J Immunol. 1990; 144:1689–94.
29. Thompson HSG, Harper N, Bevan DJ, Staines NA. Gastric administration of type II collagen delays the onset and severity of collageninduced arthritis in rats. Clin Exp Immunol. 1986; 6:581–6.
31. Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000; 164:3596–9.

32. Mellor AL, Munn DH. Tryptophan catabolism and regulation of adaptive immunity. J Immunol. 2003; 170:5809–13.

33. Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, et al. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008; 18:2483–93.

Go to : 
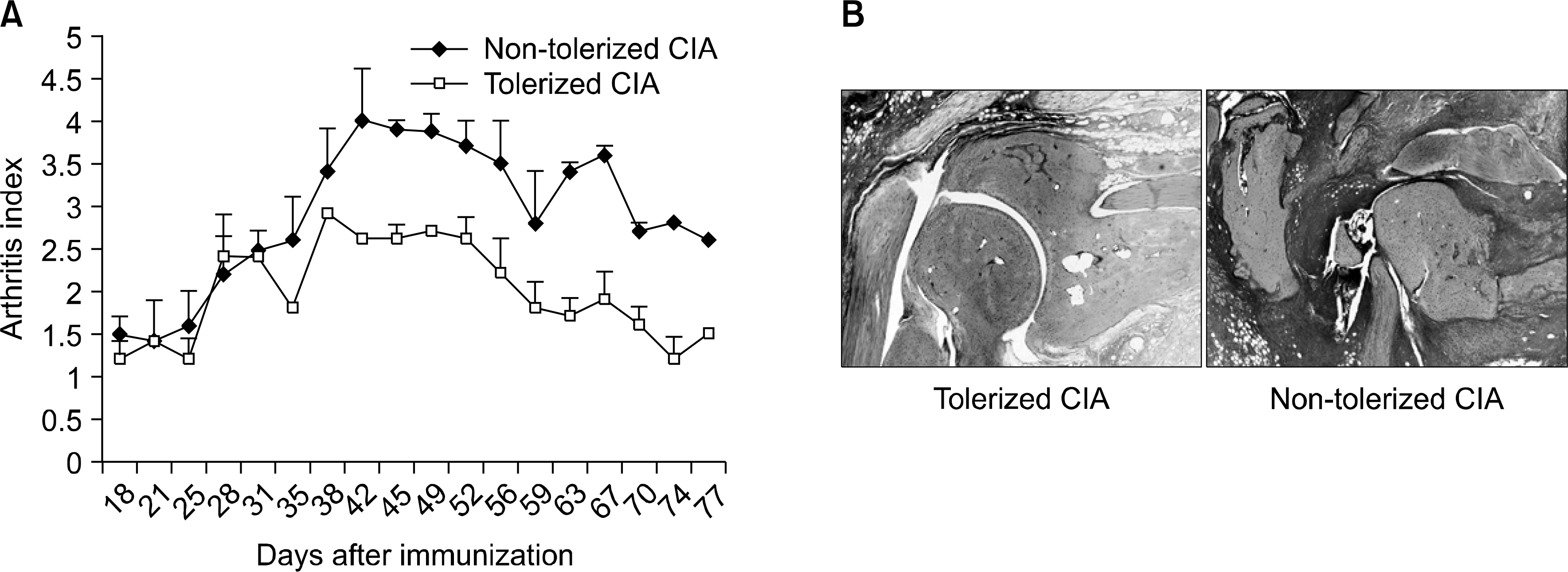 | Fig. 1.Supression of arthritis development in tolerized CIA mice. (A) Severity of arthritis was recorded as the mean arthritic index on a 0 to 4 scale according to the following criteria: 0=no edema or swelling, 1=slight edema and erythema limited to the foot or ankle, 2=slight edema and erythema from the ankle to the tarsal bone, 3=moderate edema and erythema from the ankle to the tarsal bone, and 4=edema and erythema from the ankle to the entire leg. The sum of the values from three legs, excluding the hind leg into which CII-incomplete Freund's adjuvant was injected, were determined and divided by three to obtain an average. The final value represents the average recorded by three independent observers. The arthritis index was significantly lower in the orally tolerized CIA mice group than those in the non-tolerized CIA mice group throughout the experiment period. (B) Section of joint from tolerized CIA mice and non-tolerized CIA mice were stained by hematoxylin and eosin. Tolerized CIA mice had preserved joint histology with mild inflammatory infiltrate (left), whereas non-tolerized CIA mice had intense inflammatory infiltrates and severe bony destruction (right). |
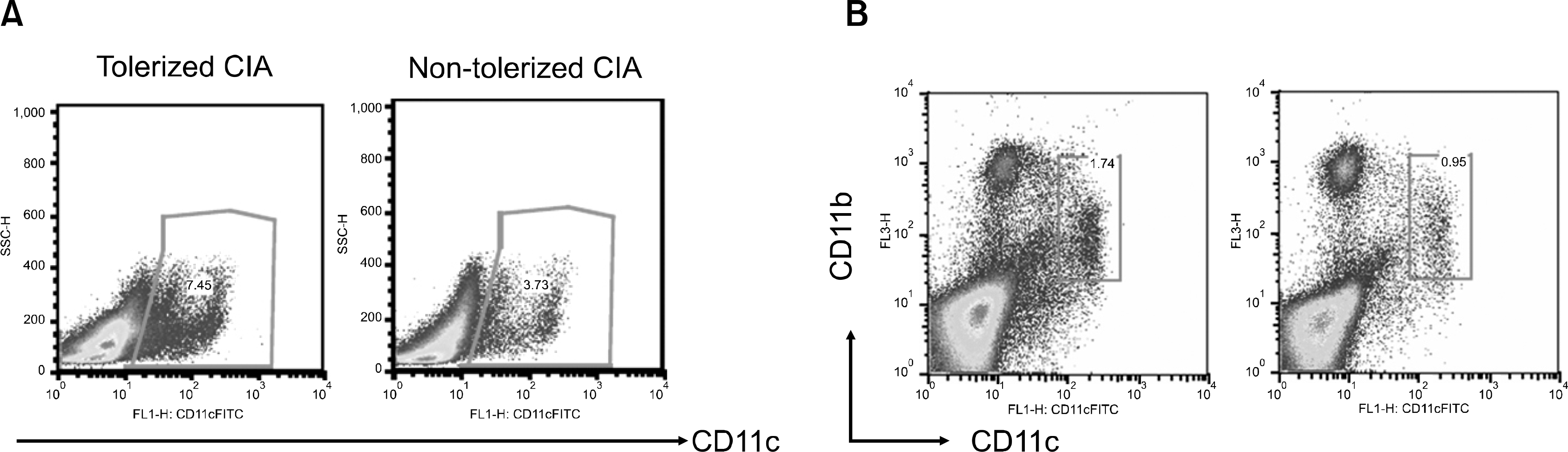 | Fig. 2.FACs analysis of DCs in splenocytes. (A) splenocytes from tolerized CIA mice and non-tolerized CIA mice were stained with anti-mouse CD11c (FITC conjugated), anti-mouse CD11b (PerCP conjugated) antibodies and were analyzed on FACs. The proportion of CD11c+ DCs in spleen was slightly higher in tolerized CIA mice than in non-tolerized CIA mice. (B) The proportion of CD11c+CD11b+ DCs in spleen was slightly higher in tolerized CIA mice than in non-tolerized CIA mice. |
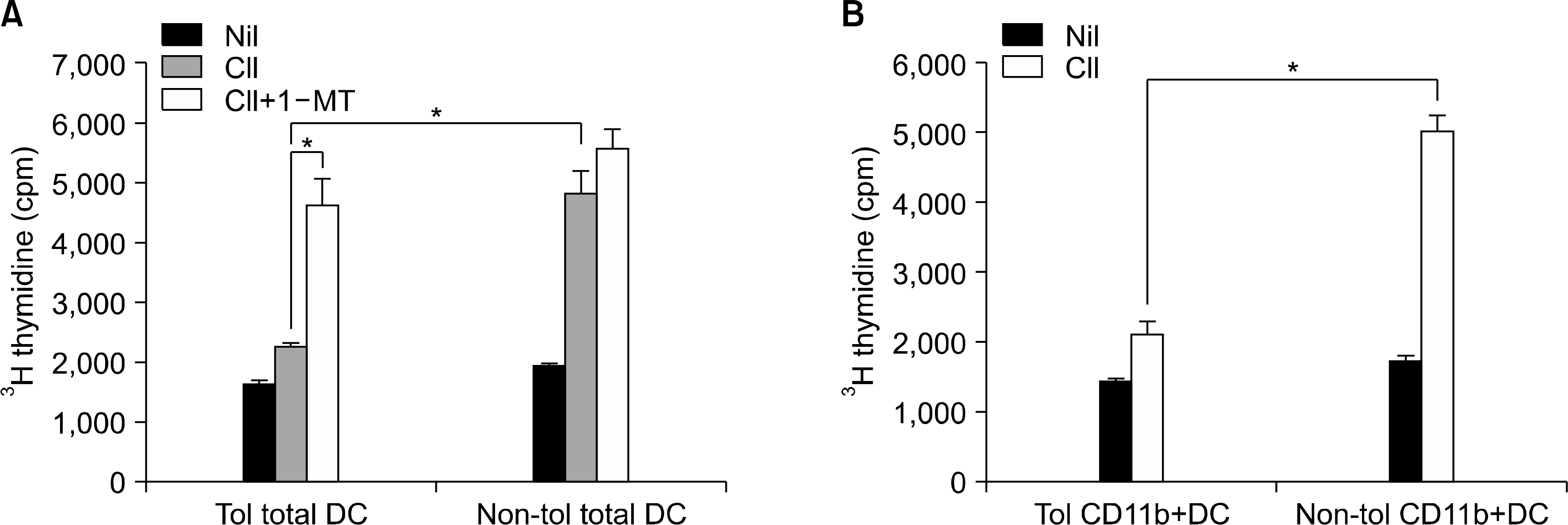 | Fig. 3.CD11c+ DCs from tolerized CIA mice suppress CII-induced T cell proliferation. (A) Splenic CD4+ T cells from non–tolerized CIA mice were cultured with splenic CD11c+ DCs from non-tolerized CIA mice or tolerized CIA mice in the presence or absence of CII (40 ug/ml) with or without pretreatment with IDO inhibitor, 1-MT. CD11c+DCs from tolerized CIA mice suppressed CII-induced proliferation of CII-reactive CD4+ T cells, which was partly abrogated by 1-MT. Values are the mean±standard deviation from three independent experiments. ∗p<0.05. (B) Splenic CD4+T cells from non – tolerized CIA mice were cultured with CD11c+CD11b+ DCs from non-tolerized CIA or tolerized CIA mice in the presence or absence of CII (40 ug/ml). CD11b+ DC from tolerized CIA mice suppressed CII-induced proliferation of CII-reactive CD4+ T cells. Values are the mean±standard deviation from three independent experiments. ∗p<0.05. |
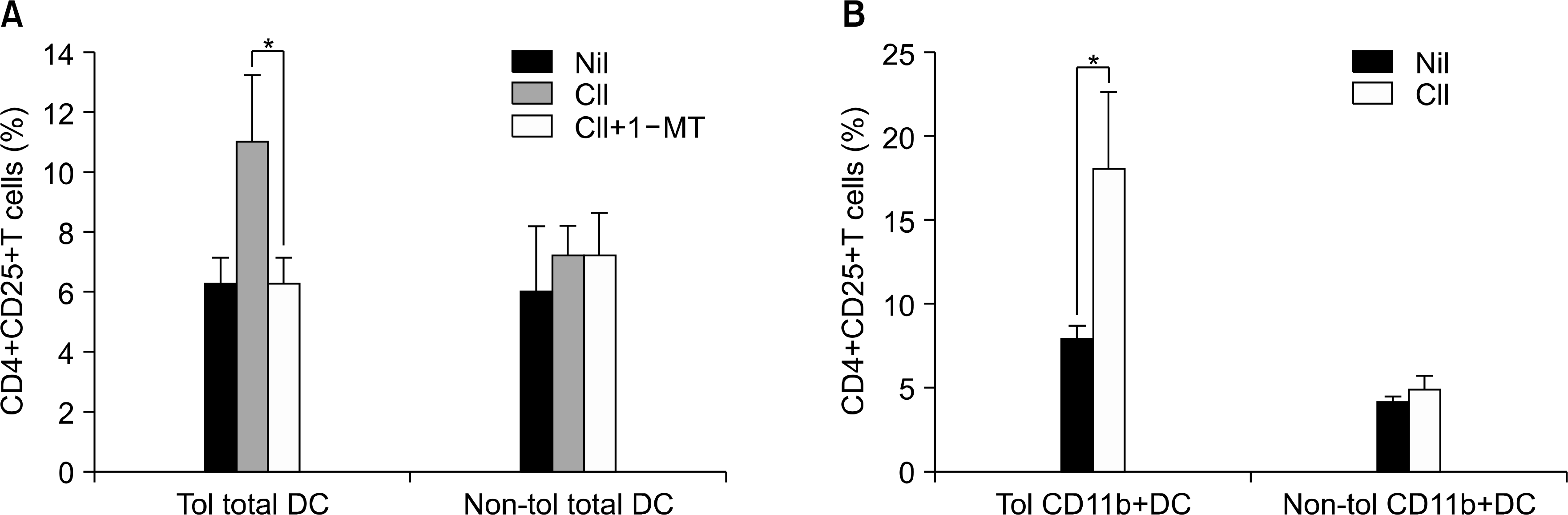 | Fig. 4.Antigen-specific CD4+CD25+ regulatory T-cell induction by DCs. (A) For regulatory T-cell induction, isolated CD4+ T cells from spleen of tolerized CIA mice were cultured with CD11c+ DCs from spleen of tolerized and non-tolerized CIA mice in the absence or presence of CII (40 μg/ml) with or without IDO inhibitor, 1-MT for 3 days. The proportion of CD4+CD25+ T cells was determined using FACs. CD4+CD25+ regulatory T cells of CD11c+ DCs in tolerized CIA mice increased, which was partly abrogated by 1-MT. Values are the mean±standard deviation from three independent experiments. ∗P<0.05. (B) CD4+CD25+ regulatory Tcell expression in the same method as (A). The numbers were represented CD4+CD25+ cell percentage by CD11c+ and CD11b+ cells. CD4+CD25+ T cells were expanded to CD11c+ CD11b+ DCs of spleen from tolerized CIA mice in the presence of CII antigen stimulation. Values are the mean±standard deviation from three independent experiments. ∗p<0.05. |
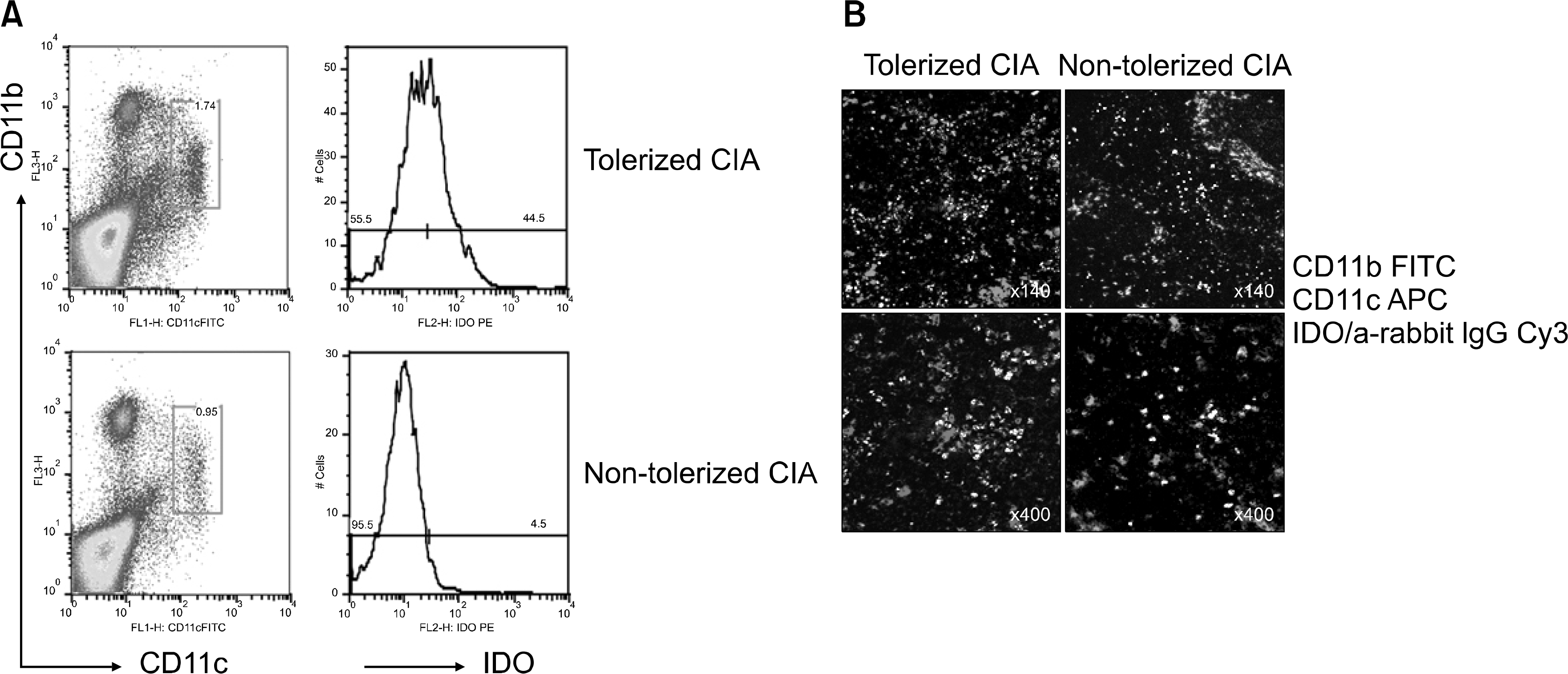 | Fig. 5.Expression of IDO on CD11c+CD11b+ DCs. (A) To experiment expression of IDO in DCs of tolerized CIA mice and non-tolerized CIA mice, splenocytes from tolerized and non-tolerized CIA mice were stained with anti mouse CD11c (FITC conjugated), anti mouse CD11b (PerCP conjugated) and IDO (PE conjugated) antibodies and were analyzed on FACs. IDO expression of CD11c+CD11b+ DCs of tolerized CIA mice increased. (B) Cryo sections of spleen from tolerized CIA mice and non-tolerized CIA mice were stained by anti mouse CD11c (APC conjugated), anti mouse CD11b (FITC conjugated) and IDO (Cy3 conjugated) antibodies. We analyzed it on confocal microscopy. Merged CD11c+ CD11b+ IDO+ cells increased in tolerized CIA mice. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download