References
2. Lard LR, Visser H, Speyer I, vander HorstBruinsma IE, Zwinderman AH, Breedveld FC, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001; 111:446–51.

3. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–24.

4. Saraux A, Berthelot JM, Chales G, Le Henaff C, Thorel JB, Hoang S, et al. Ability of the American College of Rheumatology 1987 criteria to predict rheumatoid arthritis in patients with early arthritis and classification of these patients two years later. Arthritis Rheum. 2001; 44:2485–91.

5. Dorner T, Egerer K, Feist E, Burmester GR. Rheumatoid factor revisited. Curr Opin Rheumatol. 2004; 16:246–53.
6. Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritisspecific autoantibodies. J Clin Invest. 1998; 101:273–81.

7. van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res. 2002; 4:87–93.
8. Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003; 25:1106–18.

9. Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000; 43:155–63.

10. Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007; 146:797–808.

11. Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2006; 65:845–51.

12. Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002; 21:1525–37.

13. Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2002; 21:1237–56.

14. Davey SG, Egger M. Meta-analyses of randomised controlled trials. Lancet. 1997; 350:1182.
15. Higgins JP, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med. 2002; 21:1539–58.

16. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for metaanalysis of test accuracy data. BMC Med Res Methodol. 2006; 6:31.

17. Choi SW, Lim MK, Shin DH, Park JJ, Shim SC. Diagnostic performances of anti-cyclic citrullinated peptides antibody and antifilaggrin antibody in Korean patients with rheumatoid arthritis. J Korean Med Sci. 2005; 20:473–8.

18. Kim KH, Lee SW, Chung WT. Association of anti-cyclic citrullinated peptide antibodies and functional status in rheumatoid arthritis. J Korea Rheumatism Ass. 2006; 13:46–51.
19. Kim KH, Kwon JA, Kim YK. Diagnostic performance of the anti-cyclic citrullinated peptide antibodies and rheumatoid factor isotypes in rheumatoid arthritis. J Lab Med Qual Assur. 2005; 27:195–202.
20. Kang HJ, Seo YI, Lee YK, Cho HC. Diagnostic usefulness of the anti-cyclic citrullinated peptide antibodies for rheumatoid arthritis. J Korea Rheumatism Ass. 2003; 10:117–25.
21. Song JS, Park GB, Park AJ. Comparison of anti-mutated citrullinated vimentin with anti-cyclic citrullinated peptide and rheumatoid factors for the diagnostic value of rheumatoid arthritis. Assessment. J Korea Rheumatism Ass. 2007; 14:235–41.
22. Park SH, Kim JY, Kim SK, Choe JY, Kim SK, Shin IH. Diagnostic significance of anti-CCP antibody in Korean early rheumatoid arthritis. J Korea Rheumatism Ass. 2007; 14:227–34.

23. Kim HR, Shin JW, Lee JN. Evaluation of the usefulness of anti-cyclic citrullinated peptide antibodies measured by an automated enzyme immunoassay. J Lab Med Qual Assur. 2005; 27:183–8.
24. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997; 3157:1533–7.

25. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003; 56:1129–35.

26. Berglin E, Padyukov L, Sundin U, Hallmans G, Stenlund H, Van Venrooij WJ, et al. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA-DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis. Arthritis Res Ther. 2004; 6:R303–8.
27. Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, et al. The major synovial targets of the rheumatoid arthritisspecific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001; 166:4177–84.
Go to : 
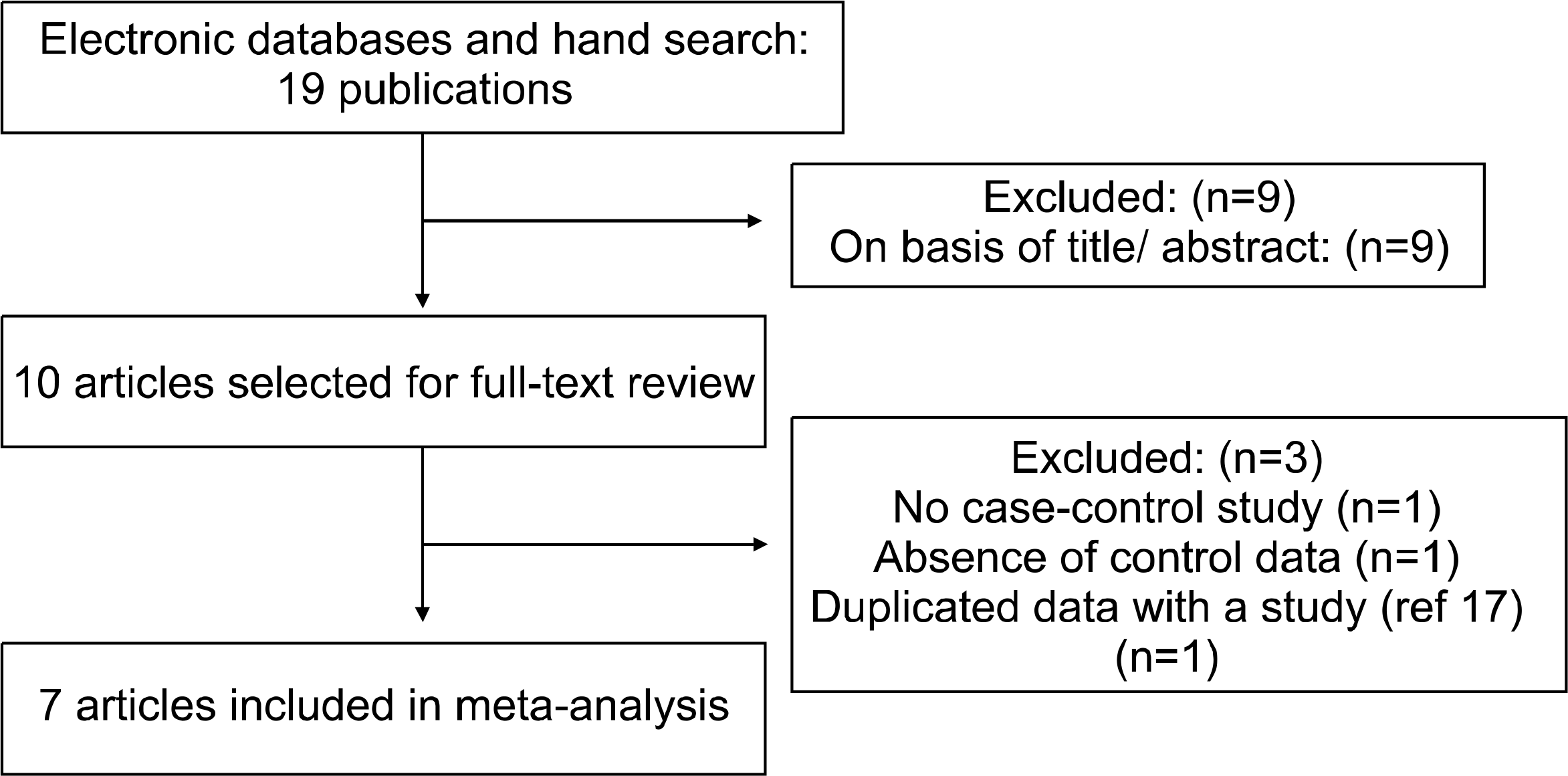 | Fig. 1.Flow diagram of study selection. Articles reported the diagnostic value of anti-CCP antibody and RF testings for RA. |
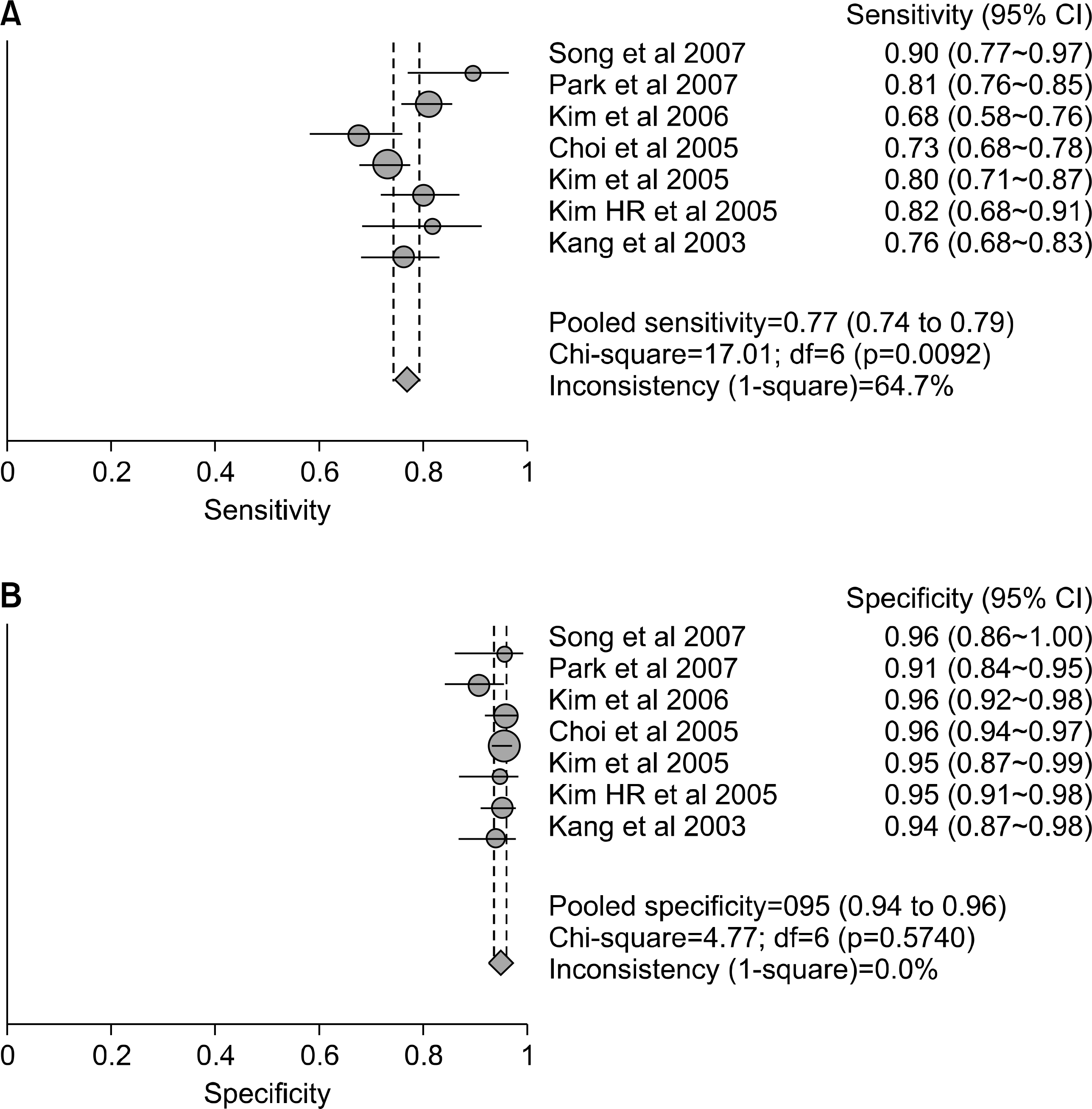 | Fig. 2.Sensitivity (A) and specificity (B) estimates for anti-CCP antibody for the diagnosis of RA. Circles and lines represent point estimates and 95% CIs, respectively. Circle areas represent relative study sizes. 1 means 100% in sensitivity and specificity. |
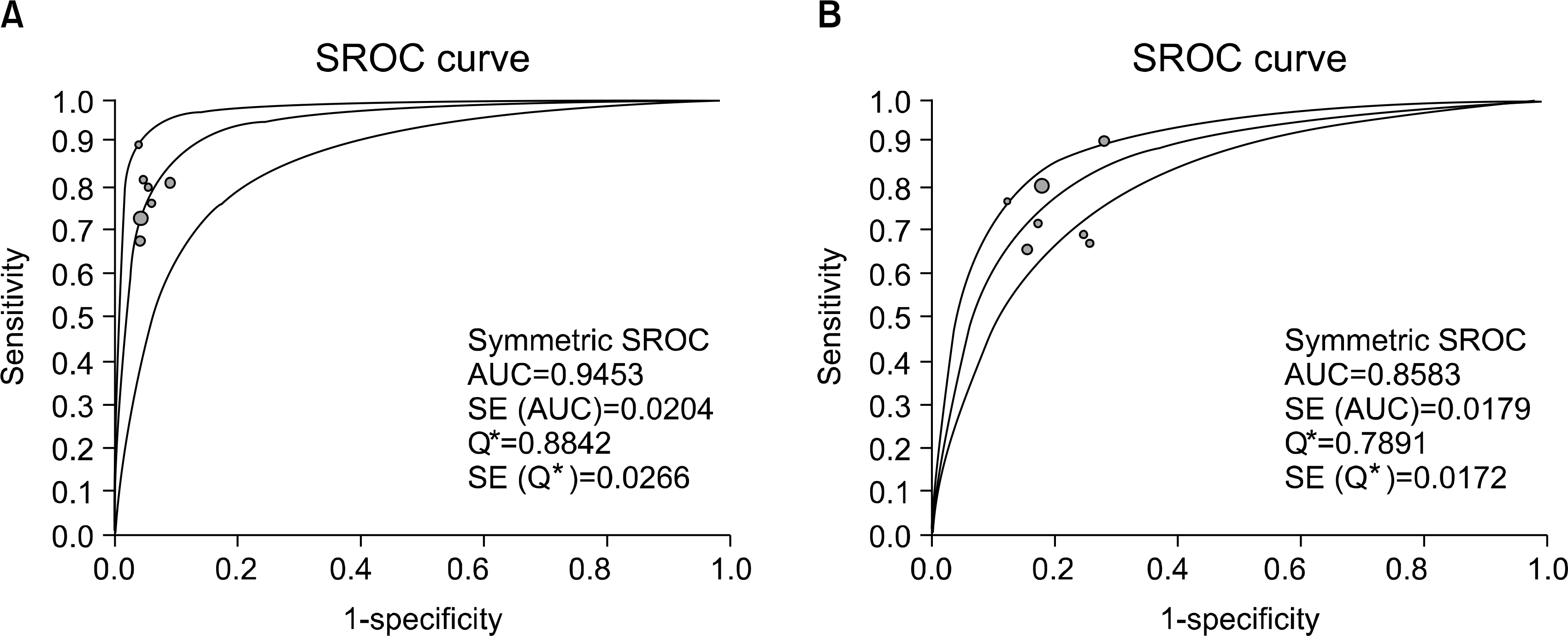 | Fig. 3.SROC curves for anti-CCP antibody (A) and for RF (B) for the diagnosis of RA Solid circles represent individual studies included in this meta-analysis. The curve shown is a regression line that summarizes overall diagnostic accuracy. SE (AUC), standard error of AUC, Q∗, an index defined by the point on the SROC curve where the sensitivity and specificity are equal; SE (Q∗), Q∗ index standard error. |
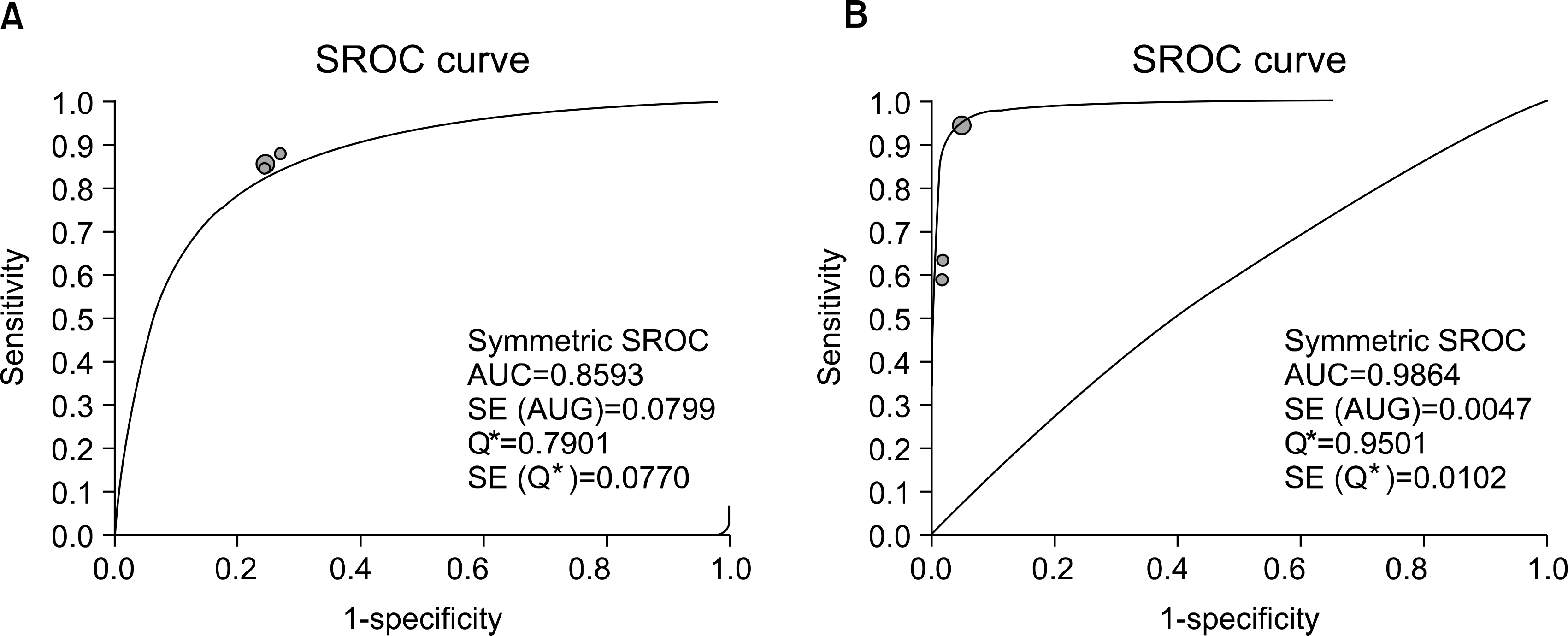 | Fig. 4.SROC curves for either anti-CCP or RF (A) and both anti-CCP and RF positivity (B) for the diagnosis of RA Solid circles represent individual studies included in this meta-analysis. The curve shown is a regression line that summarizes overall diagnostic accuracy. SE (AUC), standard error of AUC, Q∗, an index defined by the point on the SROC curve where the sensitivity and specificity are equal; SE (Q∗), Q∗ index standard error. |
Table 1.
Characteristics of individual studies included in meta-analysis
| Author | Year | Numbers | Mean age, yrs (range) | Female (%) | Anti-CCP | RF | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RA | Control | RA | Control | RA | Control | Sensitivity∗ | Specificity∗ | Sensitivity∗ | Specificity | ||
| Song et al | 2007 | 48 | OA 50 | 52.2 | 51.0 | 89.6 | 86 | 0.896(0.773∼0.965) | 0.960 (0.863∼0.995) | 0.771 (0.627∼0.880) | 0.880 (0.757∼0.99 |
| Park et al | 2007 | 262 | NRA 122 | NA | NA | 80.9 | 73.8 | 0.809(0.756∼0.855) | 0.910 (0.844∼0.954) | 0.908 (0.867∼0.940) | 0.721 (0.633∼0.79 |
| Kim et al | 2006 | 114 | NRA 202 | 51.1 (23∼80) | NA | 83.3 | NA | 0.675(0.581∼0.760) | 0.960 (0.923∼0.983) | 0.658 (0.563∼0.744) | 0.847 (0.789∼0.89 |
| Choi et al | 2005 | 324 | NRA/HC 251/28 | 51 (22∼83) | 53.4 (4∼90)/ 50.4 (1∼72) | 84.9 | 83.7/ 53.8 | 0.728 (0.676∼0.776) | 0.957 (0.936∼0.973) | 0.806 (0.758∼0.847) | 0.821 (0.786∼0.85 |
| Kim et al | 2005 | 110 | NRA/HC 30/46 | 50.0 (17∼78) | NA | 81.8 | NA | 0.800(0.713∼0.870) | 0.947 (0.871∼0.985) | 0.718 (0.624∼0.800) | 0.829 (0.725∼0.90 |
| Kim H et al | R 2005 | 49 | NRA/HC 104/51 | NA | NA | NA | NA | 0.816(0.680∼0.912) | 0.955 (0.909∼0.982) | 0.964 (0.546∼0.817) | 0.755 (0.679∼0.82 |
| Kang et al | 2003 | 134 | NRA/HC 53/33 | NA | NA | NA | NA | 0.761(0.680∼0.831) | 0.942 (0.870∼0.981) | 0.672 (0.585∼0.750) | 0.744 (0.639∼0.83 |
| Total | 1,041 | 970 | |||||||||
Table 2.
Summary results of meta-analysis in RA vs. NRA
Table 3.
Summary results of meta-analysis in RA vs. HC




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download