Abstract
Objective
βig-h3 is an extracellular matrix protein, which is overexpressed in synovial tissues of rheumatoid arthritis (RA) similar to adhesive glycoproteins. We sought to evaluate the compensatory role of βig-h3 with adhesive glycoproteins in mediating the adhesion of fibroblast-like synoviocytes (FLS) and to confirm the inhibitory effect of YH18 peptide of the 2nd fas-1 domain in βig-h3-mediated adhesion.
Methods
The adhesion of FLS isolated from synovial tissues of RA, was evaluated in 96 well microtiter plate coated with matrix proteins. Inhibitory effect of YH18 peptides from the 2nd and 4th fas-1 domains was estimated in βig-h3-mediated adhesion of FLS.
Results
The adhesion of FLS on βig-h3 was weaker than that of fibronectin and vitronectin. The βig-h3-mediated adhesion was enhanced by the stimulation with phorbol myristate acetate (PMA), but not by cytokines and growth factors. Combination of fibronectin with βig-h3 synergistically enhanced the adhesion of FLS, in contrast to the additive effect of vitronectin combined with βig-h3. YH18 peptide of the 2nd fas-1 domain did not block the βig-h3-mediated adhesion of FLS.
References
1. Knedla A, Neumann E, Muller-Ladner U. Developments in the synovial biology field 2006. Arthritis Res Ther. 2007; 9:209.

2. Barilla ML, Carsons SE. Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum. 2000; 29:252–65.

3. Tomasini-Johansson BR, Milbrink J, Pejler G. Vitronectin expression in rheumatoid arthritic synovia-inhibition of plasmin generation by vitronectin produced in vitro. Br J Rheumatol. 1998; 37:620–9.

4. Lavietes BB, Carsons S, Diamond HS, Laskin RS. Synthesis, secretion, and deposition of fibronectin in cultured human synovium. Arthritis Rheum. 1985; 28:1016–26.

5. Petrow PK, Hummel KM, Schedel J, Franz JK, Klein CL, Muller-Ladner U, et al. Expression of osteopontin messenger RNA and protein in rheumatoid arthritis: effects of osteopontin on the release of collagenase 1 from articular chondrocytes and synovial fibroblasts. Arthritis Rheum. 2000; 43:1597–605.

6. Liao HX, Haynes BF. Role of adhesion molecules in the pathogenesis of rheumatoid arthritis. Rheum Dis Clin North Am. 1995; 21:715–40.

7. Liaw L, Almeida M, Hart CE, Schwartz SM, Giachelli CM. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ Res. 1994; 74:214–24.

8. Bae JS, Lee SH, Kim JE, Choi JY, Park RW, YongPark J, et al. Betaig-h3 supports keratinocyte adhesion, migration, and proliferation through alpha3-beta1 integrin. Biochem Biophys Res Commun. 2002; 294:940–8.
9. Kim JE, Kim SJ, Lee BH, Park RW, Kim KS, Kim IS, et al. Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-beta-induced gene, betaig-h3. J Biol Chem. 2000; 275:30907–15.
10. Park SW, Bae JS, Kim KS, Park SH, Lee BH, Choi JY, et al. Beta ig-h3 promotes renal proximal tubular epithelial cell adhesion, migration and proliferation through the interaction with alpha3beta1 integrin. Exp Mol Med. 2004; 36:211–9.
11. Nam JO, Kim JE, Jeong HW, Lee SJ, Lee BH, Choi JY, et al. Identification of the alphavbeta3 integrin-interacting motif of betaig-h3 and its antiangiogenic effect. J Biol Chem. 2003; 278:25902–9.
12. Kim JE, Jeong HW, Nam JO, Lee BH, Choi JY, Park RW, et al. Identification of motifs in the fasciclin domains of the transforming growth factor-betainduced matrix protein betaig-h3 that interact with the alphavbeta5 integrin. J Biol Chem. 2002; 277:46159–65.
13. Kang YM, Kim SI, Kim JS, You DW, Sa KH, Park EJ, et al. Regulation of βig-h3 Production in Rheumatoid Synovitis by Inflammatory Mediators. J Korean Rheum Assoc. 2005; 12:73–82.
14. Nam EJ, Sa KH, You DW, Cho JH, Seo JS, Han SW, et al. Upregulated transforming growth factor beta-inducible gene h3 in rheumatoid arthritis mediates adhesion and migration of synoviocytes through alpha v beta3 integrin: Regulation by cytokines. Arthritis Rheum. 2006; 54:2734–44.
15. Thapa N, Kang KB, Kim IS. Betaig-h3 mediates osteoblast adhesion and inhibits differentiation. Bone. 2005; 36:232–42.
16. Kim JE, Kim SJ, Jeong HW, Lee BH, Choi JY, Park RW, et al. RGD peptides released from beta ig-h3, a TGF-beta-induced cell-adhesive molecule, mediate apoptosis. Oncogene. 2003; 22:2045–53.
17. Munier FL, Korvatska E, Djemai A, LePaslier D, Zografos L, Pescia G, et al. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet. 1997; 15:247–51.

18. O'Brien ER, Bennett KL, Garvin MR, Zderic TW, Hinohara T, Simpson JB, et al. Beta ig-h3, a transforming growth factor-beta-inducible gene, is overexpressed in atherosclerotic and restenotic human vascular lesions. Arterioscler Thromb Vasc Biol. 1996; 16:576–84.
19. Lee SH, Bae JS, Park SH, Lee BH, Park RW, Choi JY, et al. Expression of TGF-beta-induced matrix protein betaig-h3 is up-regulated in the diabetic rat kidney and human proximal tubular epithelial cells treated with high glucose. Kidney Int. 2003; 64:1012–21.
20. Ferguson JW, Mikesh MF, Wheeler EF, LeBaron RG. Developmental expression patterns of Beta-ig (betaIG-H3) and its function as a cell adhesion protein. Mech Dev. 2003; 120:851–64.
21. Skonier J, Neubauer M, Madisen L, Bennett K, Plowman GD, Purchio AF, et al. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol. 1992; 11:511–22.
22. Andreakos ET, Foxwell BM, Brennan FM, Maini RN, Feldmann M. Cytokines and anticytokine biologicals in autoimmunity: present and future. Cytokine Growth Factor Rev. 2002; 13:299–313.

24. Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest. 1996; 97:2139–44.

25. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002; 110:673–87.
26. Kim JE, Park RW, Choi JY, Bae YC, Kim KS, Joo CK, et al. Molecular properties of wild-type and mutant betaIG-H3 proteins. Invest Ophthalmol Vis Sci. 2002; 43:656–61.
27. Hogg N, Henderson R, Leitinger B, McDowall A, Porter J, Stanley P, et al. Mechanisms contributing to the activity of integrins on leukocytes. Immunol Rev. 2002; 186:164–71.

28. Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000; 275:21785–8.

30. Borges E, Jan Y, Ruoslahti E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J Biol Chem. 2000; 275:39867–73.
31. Rinaldi N, Barth T, Henne C, Mechterscheimer G, Moller P. Synoviocytes in chronic synovitis in situ and cytokine stimulated synovial cells in vitro neo-express alpha 1, alpha 3 and alpha 5 chains of beta 1 integrins. Virchows Arch. 1994; 425:171–80.

32. Defilippi P, Truffa G, Stefanuto G, Altruda F, Silengo L, Tarone G, et al. Tumor necrosis factor alpha and interferon gamma modulate the expression of the vitronectin receptor (integrin beta 3) in human endothelial cells. J Biol Chem. 1991; 266:7638–45.

33. Shimizu Y, Van Seventer GA, Horgan KJ, Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990; 345:250–3.
Fig. 1.
Comparison of βig-h3, vitronectin, and fibronectin in adhesion of human FLS. FLS were seeded onto surfaces of 96-well culture plates which were precoated with different concentrations (0.1 ug/mL, 1 ug/mL and 10 ug/mL) of βig-h3, vitronectin, or fibronectin and then were incubated for 2 hours at 37oC. After seeding and incubation, FLS attached to the surfaces were quantified by hexosaminidase assay. Values are presented as the mean±SEM of triplicate experiments.
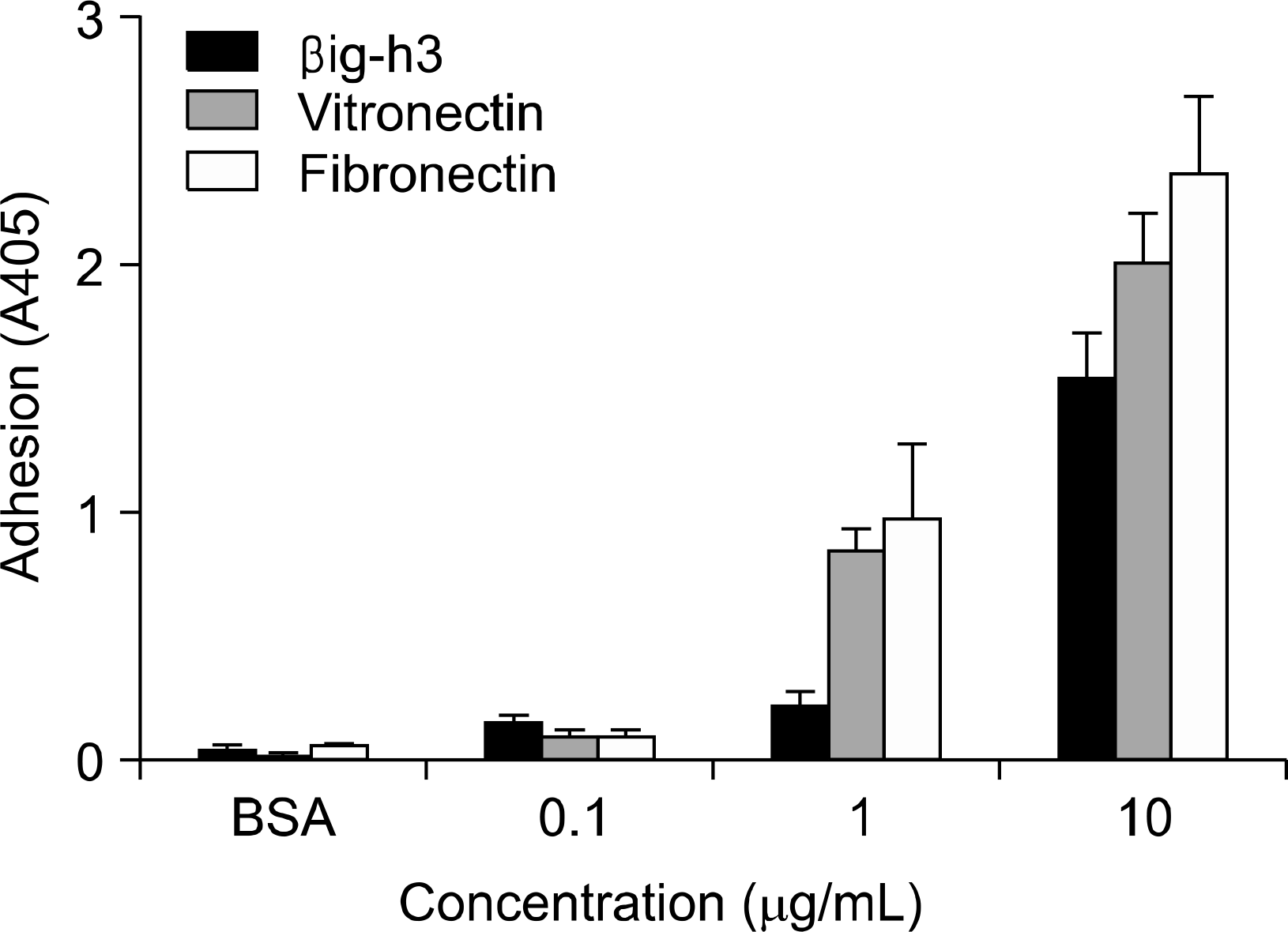
Fig. 2.
Regulation of βig-h3-mediated adhesion of FLS by cytokines, growth factors, and nonspecific stimulants. FLS were treated with different cytokines (recombinant human TNF-α, IL-1β, IL-6, IL-4, IL-10, IFN-γ, and TGF-β1), growth factors (VEGF, IGF-1, EGF, PDGF-BB, and FGF-basic), and nonspecific cell stimulants (PMA and LPS) for 30 minutes at 37oC. Pretreated FLS were added to the coated wells with 5 ug/mL of βig-h3 and were incubated for 2 hours at 37°C. After seeding and incubation, FLS attached to the surfaces were quantified by hexosaminidase assay. TGF-β1, 5 ng/mL; TNF-α, IL-1β, IL-4, IL-6, IL-10, VEGF, IGF-1 and EGF, 10 ng/mL; PDGF-BB, FGF-basic, and IFN-γ, 100 ng/mL; PMA, 60 ng/mL; and LPS 5 μg/mL. Values are presented as the mean±SEM of triplicate experiments.
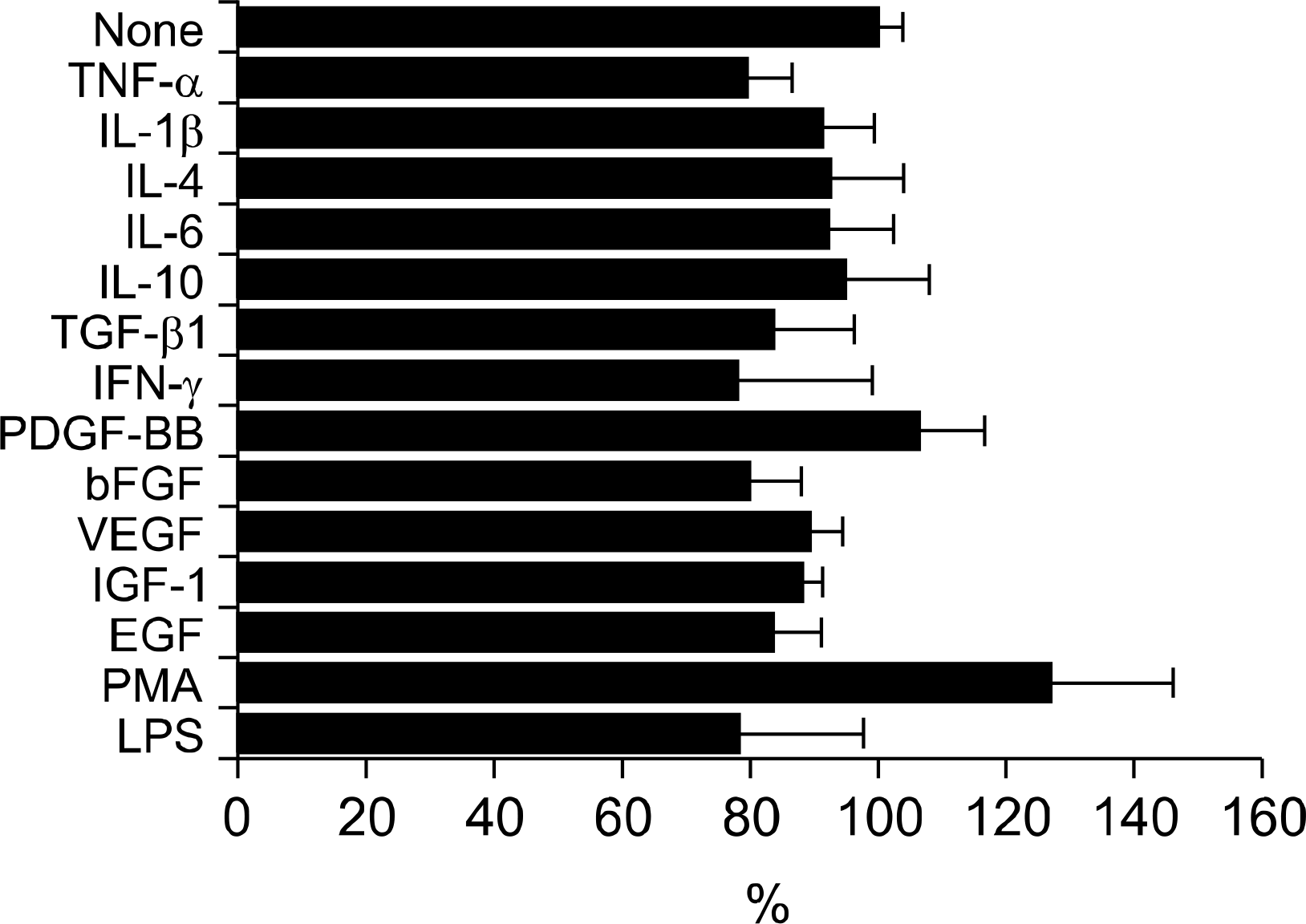
Fig. 3.
Compensatory role of βig-h3 with matrix proteins in mediating adhesion of FLS. FLS were added to the precoated 96-well culture plates coated with (A) βig-h3 and fibronectin or (B) βig-h3 and vitronectin, and were incubated for 2 hours at 37oC. Low concentration (0.1 ug/mL) of fibronectin, 1 ug/mL of vitronectin, and different concentrations (0.1 ug/mL, 1 ug/mL, and 10 ug/mL) of βig-h3 were used. After incubation, FLS attached to the surfaces were quantified. Values are presented as the mean±SEM of, at least, 3 independent experiments.
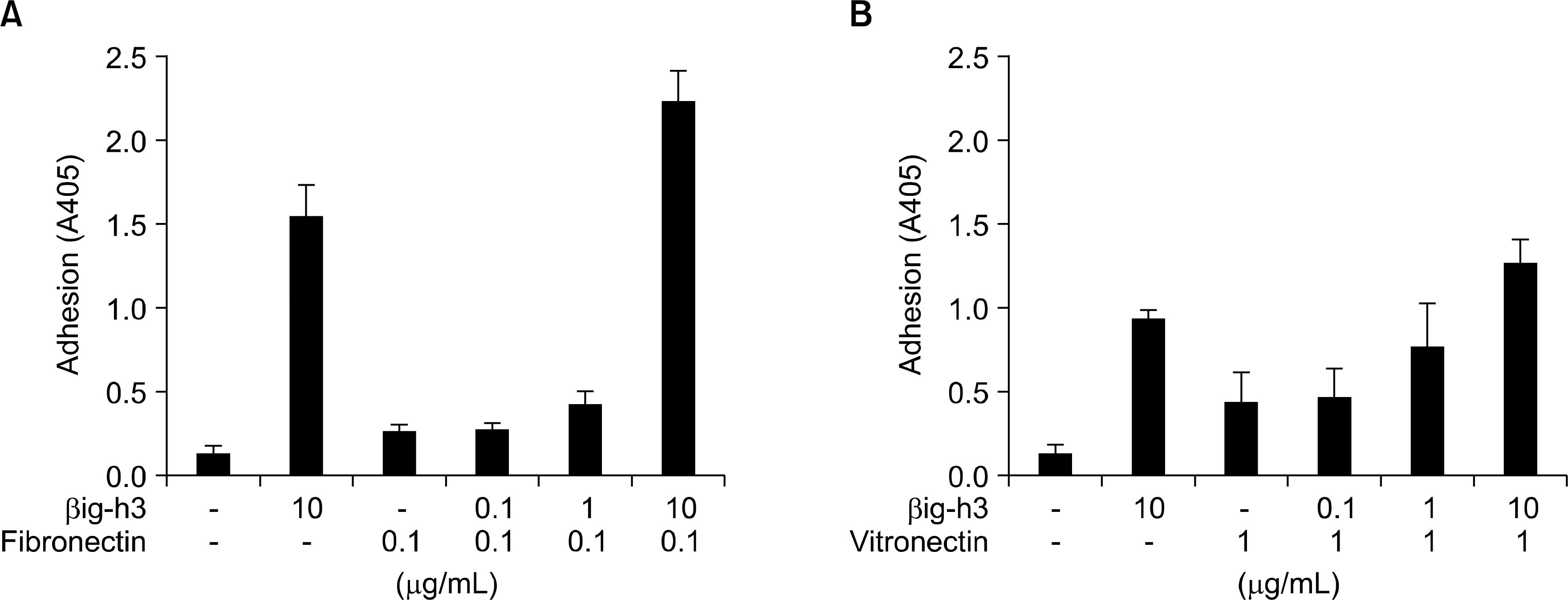
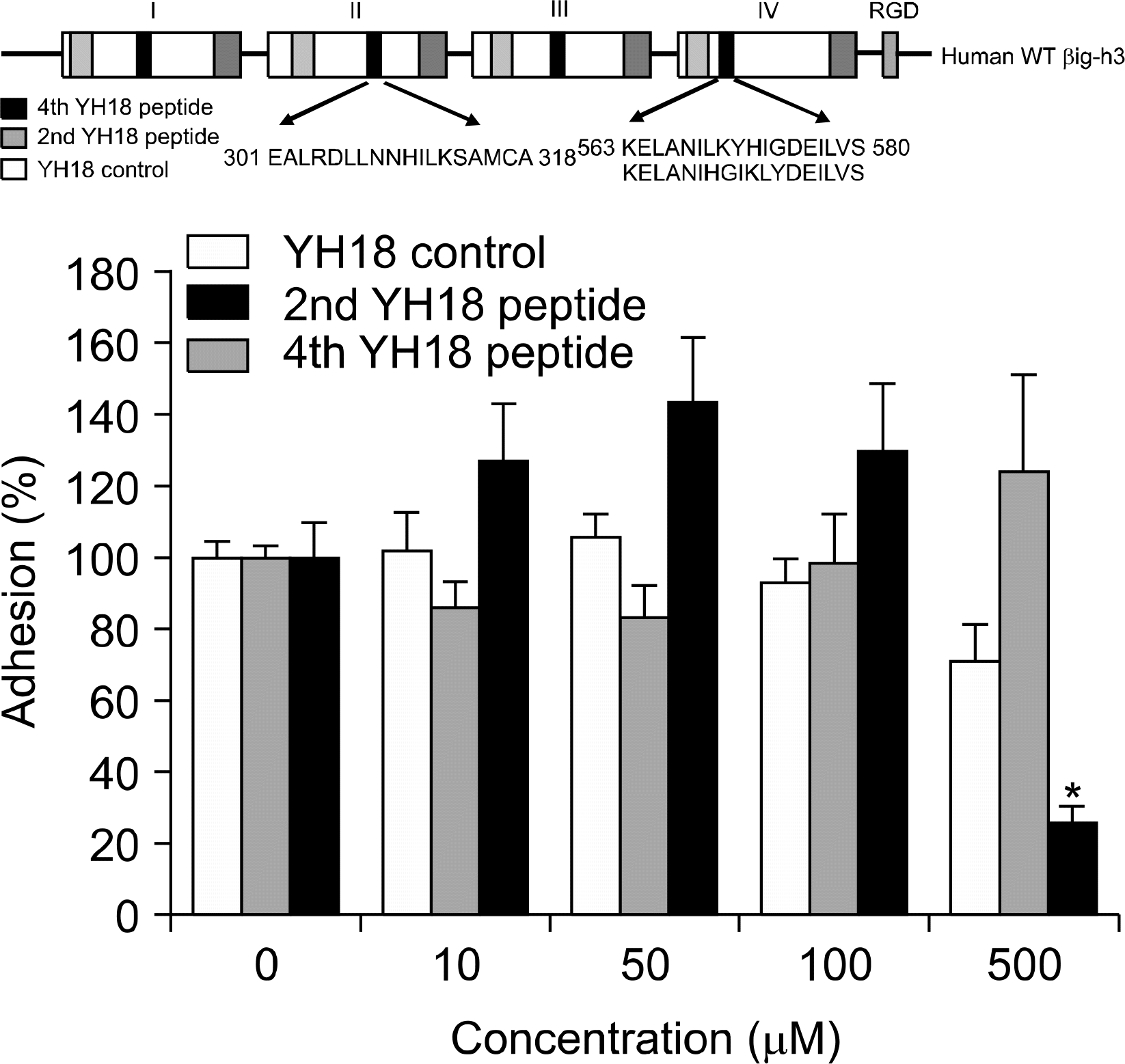
Fig. 4.
Identification of the motif mediating FLS adhesion to βig-h3. The 2nd YH18 peptide (spanning amino acids 301∼318 in the 2nd fas-1 domain), 4th YH18 peptide (spanning amino acids 563∼580 in the 4th fas-1 domain), and YH18 control peptide (containing the amino acids of the 4th YH18 peptide but in a scrambled order) were prepared. FLS were preincubated with the YH18 control, 2nd YH18, or 4th YH18 peptides at different concentrations (0, 10, 50, 100, and 500 uM) for 30 minutes at 37oC and then added to the βig-h3-coated wells. Values are presented as the mean±SEM of at least 3 independent experiments. ∗p<0.05, 4th YH18 peptide versus YH18 control peptide.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download