Abstract
Objective
To define the state of remission based on American College of Rheumatology (ACR) preliminary criteria in Korean patients with rheumatoid arthritis (RA).
Methods
A hundred three patients of RA, followed up over 1 year, were selected at Ewha medical center from May 2000 to May 2006. Remission was defined by ACR preliminary criteria. Data were obtained from the initial and the last visit. Data on initial tender joint count (TJC) and swollen joint count (SJC), treatment, disease duration, remission duration were collected. Initial ESR, CRP, rheumatoid factor (RF), TJC and SJC were also performed at the last clinical visit or at the time of remission.
Results
Patients in remission were 35%. The maintenance duration of remission was 4.8±9.0 (mean±SD) months. Remission group had shorter disease duration (20.2±34.7 vs. 58.2±83.2 months, p=0.010), were at earlier stage of the disease (<2 years of symptom onset) (80.6 vs. 52.2%, p=0.006) compared to non-remission group. Percentage of patients showing decrease in RF titer was significantly higher in the remission group compared to the non-remission group (p=0.049). However, seronegative conversion of RF was not related to remission status (15.6 vs. 14.8%). In the non-remission group, pain was the most persistently non-satisfying clinical variable of the ACR preliminary criteria.
References
1. Pinals RS, Masi AT, Larsen RA. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum. 1981; 24:1308–15.

2. Fuchs HA. The use of the disease activity score in the analysis of clinical trials in rheumatoid arthritis. J Rheumatol. 1993; 20:1863–6.
3. Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993; 36:729–40.
4. Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004; 363:675–81.

5. St. Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004; 50:3432–43.
6. Wolfe F, Hawley DJ. Remission in rheumatoid arthritis. J Rheumatol. 1985; 12:245–52.
7. Ferraccioli GF, Gremese E, Tomietto P, Favret G, Damato R, Di Poi E, et al. Analysis of improvements, full responses, remission and toxicity in rheumatoid patients treated with step-up combination therapy (methotrexate, cyclosporine A, sulphasalazine) or monotherapy for three years. Rheumatology (Oxford). 2002; 41:892–8.
8. Mӧttӧnen T, Hannonen P, Leirisalo-Repo M, Nissilӓ M, Kautiainen H, Korpela M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomized trial. FIN-RACo trial group. Lancet. 1999; 353:1568–73.
9. Short CL, Bauer W, Reynold WE. Rheumatoid arthritis. 222–39. Cambridge, Havard University Press,. 1957.
10. Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995; 38:727–35.

11. van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL, et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism criteria. Arthritis Rheum. 1996; 39:34–40.

12. Food and Drug Administration. Guidance for industry. Clinical development programs for drugs, devices, and biological products for the treatment of rheumatoid arthritis. US Department of Health and Human Services, FDA, February. 1999.
13. Fransen J, Creemers MCW, van Riel PLCM. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford). 2004; 43:1252–5.

14. Sesin CA, Bingham CO, III . Remission in rheumatoid arthritis: wishful thinking or clinical reality? Semin Arthritis Rheum. 2005; 35:185–96.

15. Alarcon GS, Blackburn Jr WD, Calvo A, Castaneda O. Evaluation of the American Rheumatism Association preliminary criteria for remission in rheumatoid arthritis: a prospective study. J Rheumatol. 1987; 14:93–6.
16. Balsa A, Carmona L, Gonzalez-Alvaro I, Belmonte MA, Tena X, Sanmarti R, et al. Value of Disease Activity Score 28 (DAS28) and DAS28–3 compared to American College of Rheumatology-defined remission in rheumatoid arthritis. J Rheumatol. 2004; 31:40–6.
17. Korpela M, Laasonen L, Hannonen P, Kautiainen H, Leirisalo-Repo M, Hakala M, et al. Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: five-year experience from the FIN-RACo study. Arthritis Rheum. 2004; 50:2072–81.
18. Mottonen T, Hannonen P, Korpela M, Nissila M, Kautiainen H, Ilonen J, et al. Delay to institution fo therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 2002; 46:894–8.
19. Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol. 1995; 22:639–43.
20. Belza BL, Henke CJ, Yelin EH, Epstein WV, Gillis CL. Correlates of fatigue in older adults with rheumatoid arthritis. Nurs Res. 1993; 42:93–9.

21. Kirwan JR, Hewlett SE, Heiberg T, Hughes RA, Carr M, Hehir M, et al. Incorporating the patient perspective into outcome assessment in rheumatoid arthritis-progress at OMERACT 7. J Rheumatol. 2005; 32:2250–6.
22. Prevoo ML, van Gestel AM, van't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL, et al. Remission in a prospective study of patients with rheumatoid arthritis: American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol. 1996; 35:1101–5.

23. Sokka T, Pincus T. Most patients receiving routine care for rheumatoid arthritis in 2001 did not meet inclusion criteria for most recent clinical trials or American College of Rheumatology criteria for remission. J Rheumatol. 2003; 30:1138–46.
24. Young A, Dixey J, Cox N, Davies P, Davlin J, Emery P, et al. How does functional disability in early rheumatoid arthritis (RA) affect patients and their live? Results of 5 years of follow-up in 732 patients from the Early RA study (ERAS). Rheumatology (Oxford). 2000; 39:603–11.
25. Lindqvist E, Saxne T, Geborek P, Eberhardt K. Ten year outcome in a cohort of patients with early rheumatoid arthritis: health status, disease process, and damage. Ann Rheum Dis. 2002; 61:1055–9.

26. Bukhari M, Lunt M, Harrison BJ, Scott DG, Symmons DP, Silman AJ, et al. Rheumatoid factor is the major predictor of increasing severity of radiographic erosions in rheumatoid arthritis: results from the Norfolk Arthritis Register Study, a large inception cohort. Arthritis Rheum. 2002; 46:906–12.

27. van Zeben D, Hazes JM, Zwinderman AH, Cats A, van der Voort EA, Breedveld FC, et al. Clinical significance of rheumatoid factors in early rheumatoid arthritis: results of a follow up study. Ann Rheum Dis. 1992; 51:1029–35.

28. van Schaardenburg D, Hazes JM, de Boer A, Zwinderman AH, Meijers KA, Breedveld FC. Outcome of rheumatoid arthritis in relation to age and rheumatoid factor at diagnosis. J Rheumatol. 1993; 20:45–52.
29. Alarcon GS, Schrohenloher RE, Bartolucci AA, Ward JR, Williams HJ, Koopman WJ. Suppression of rheumatoid factor production by methotrexate in patients with rheumatoid arthritis. Evidence for differential influences of therapy and clinical status on IgM and IgA rheumatoid factor expression. Arthritis Rheum. 1990; 33:1156–61.
Fig. 1.
Distribution of remission duration in remission group is shown by Kaplan-Meier analysis. Patients on remission within 12 months are 36.1% (13/36).
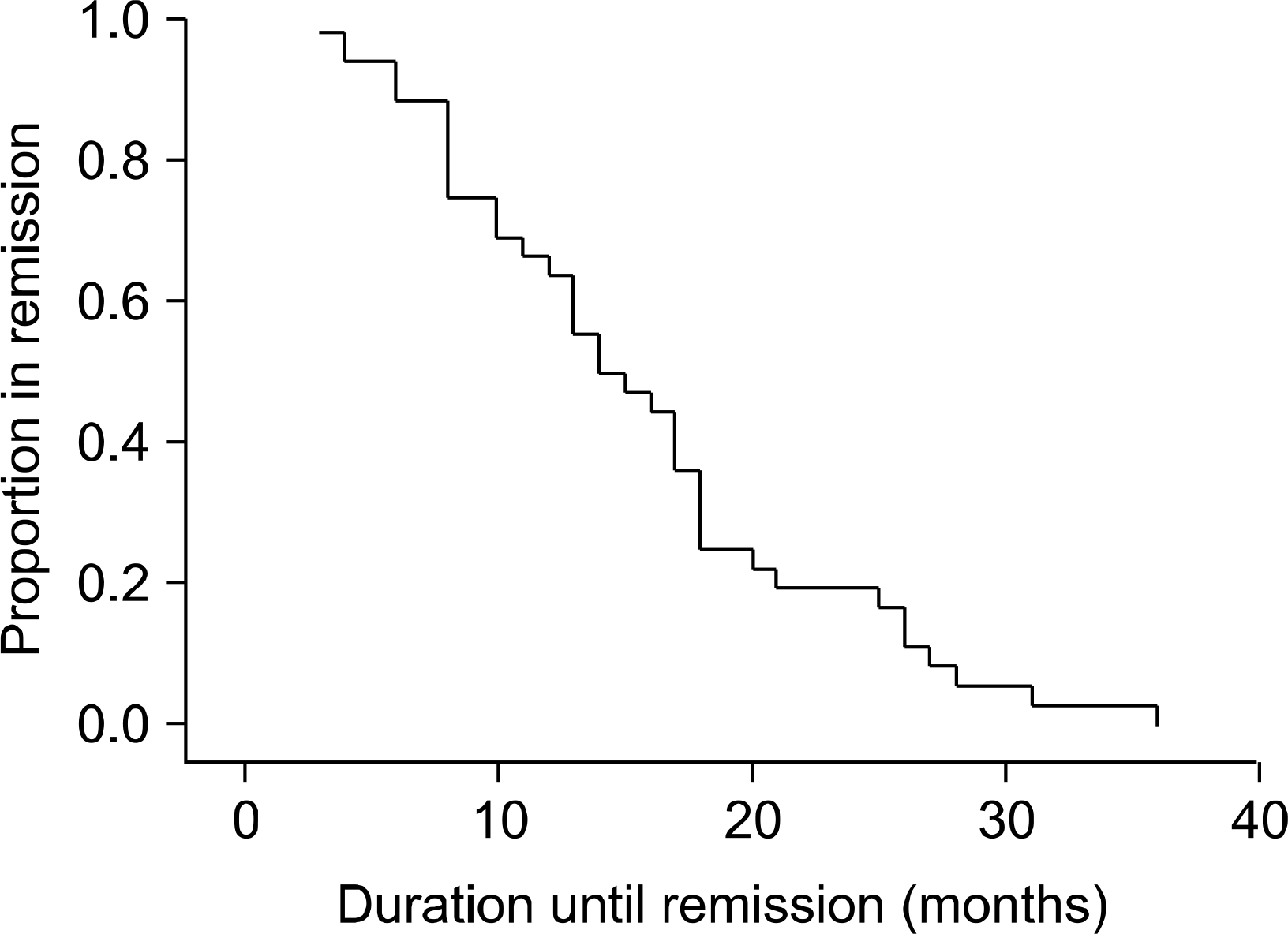
Fig. 2.
Changes in rheumatoid factor titer during the follow up period in RA remission group (A), non-remission group (B).
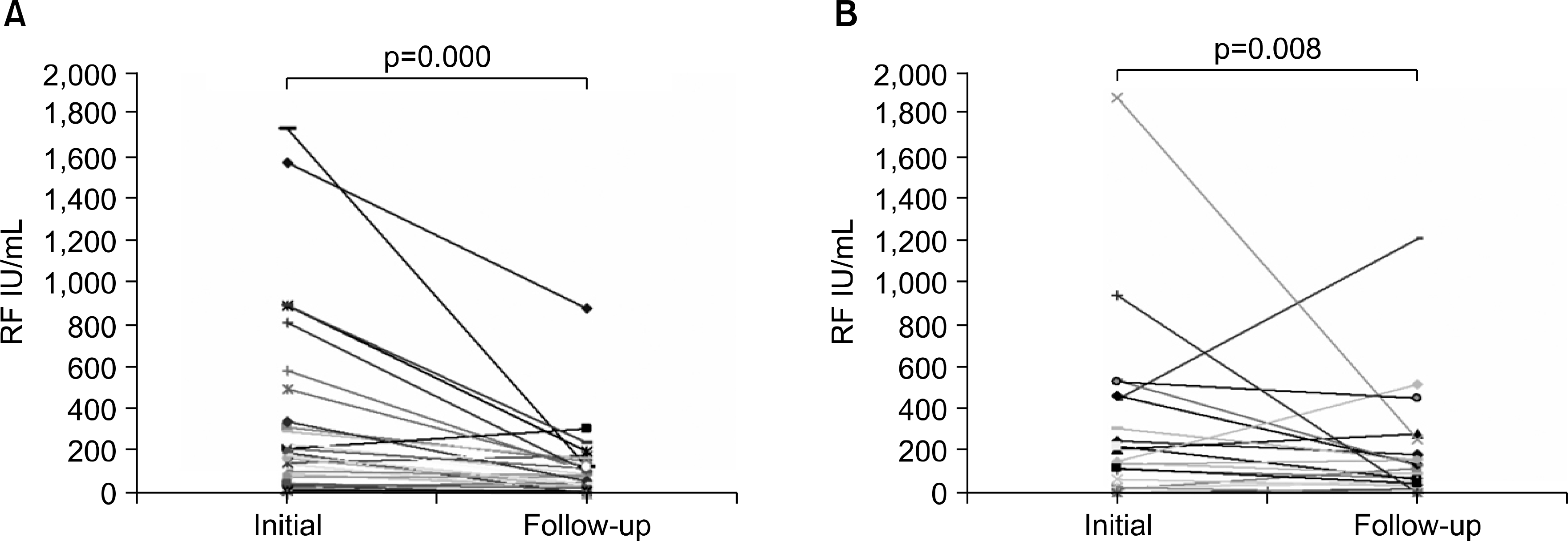
Table 1.
American College of Rheumatology preliminary criteria for remission (1)
Table 3.
Remission status of RA patients
Table 2.
Clinical and demographic characteristics of RA patients
Table 4.
Comparison of clinical and demographic characteristics of RA patients according to the state of remission
Table 5.
Comparison of rheumatoid factor changes during the follow up period in seropositive RA patients between the remission group and the nonremission group
| Remission n=32 (%) No remission n=54 (%) p |
|---|
| Rheumatoid factor decrease 27 (84.4) 34 (63) 0.049 |
| Rheumatoid factor increase 5 (15.6) 20 (37) 0.049 |
| Seronegative conversion∗ 5 (15.6) 8 (14.8) NS |
Table 6.
Distribution of ACR remission criteria in R patients who did not achieved remission follow up
| Criteria | Criteria |
|---|---|
| satisfying, | non-satisfying |
| n (%) | n (%) |
| Morning stiffness <15 min∗ 33 (49.3) | 34 (50.7) |
| No fatigue∗ 43 (64.2) | 24 (35.8) |
| No joint pain∗ 1 (1.5) | 66 (98.5) |
| No tender joint∗ 33 (49.3) | 34 (50.7) |
| No swollen joint∗ 28 (41.8) | 39 (58.2) |
| Normal ESR 53 (79.1) | 14 (20.9) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download