References
1. Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007; 19:327–36.

2. Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005; 8:25–54.
4. Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003; 197:489–501.

5. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002; 3:944–50.

6. Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007; 178:7868–78.

7. Schultze JL, Michalak S, Lowne J, Wong A, Gilleece MH, Gribben JG, et al. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon gamma, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999; 189:1–12.
8. Knoechel B, Lohr J, Kahn E, Abbas AK. The link between lymphocyte deficiency and autoimmunity: roles of endogenous T and B lymphocytes in tolerance. J Immunol. 2005; 175:21–6.
9. Subudhi SK, Alegre ML, Fu YX. The balance of immune responses: costimulation verse coinhibition. J Mol Med. 2005; 83:193–202.

10. Yamazaki T, Akiba H, Koyanagi A, Azuma M, Yagita H, Okumura K. Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-γ-induced nitric oxide production. J Immunol. 2005; 175:1586–92.
11. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001; 291:319–22.

12. Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004; 101:10691–6.

13. Tsitoura DC, Yeung VP, DeKruyff RH, Umetsu DT. Critical role of B cells in the development of T cell tolerance to aeroallergens. Int Immunol. 2002; 14:659–67.

14. Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol. 2000; 12:597–605.

15. Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994; 12:881–922.

16. Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA, et al. Expression of functional CD40 by vascular endothelial cells. J Exp Med. 1995; 182:33–40.

17. Higuchi T, Aiba Y, Nomura T, Matsuda J, Mochida K, Suzuki M, et al. Cutting edge: ectopic expression of CD40 ligand on B cells induces lupus-like autoimmune disease. J Immunol. 2002; 168:9–12.

18. Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003; 14:155–74.

19. Broxmeyer HE. Is interleukin 17, an inducible cytokine that stimulates production of other cytokines, merely a redundant player in a sea of other biomolecules? J Exp Med. 1996; 183:2411–5.

20. Kehlen A, Pachnio A, Thiele K, Langner J. Gene expression induced by interleukin-17 in fibroblast-like synoviocytes of patients with rheumatoid arthritis: upregulation of hyaluronan-binding protein TSG-6. Arthritis Res Ther. 2003; 5:186–92. R.
21. Koshy PJ, Henderson N, Logan C, Life PF, Cawston TE, Rowan AD. Interleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proinflammatory cytokines. Ann Rheum Dis. 2002; 61:704–13.

22. Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon-γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000; 192:123–8.
23. Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, et al. Defective CD4+CD25+ regulatory T cell functioning in collageninduced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-γ. Arthritis Res Ther. 2000; 7:402–15. R.
24. Wood KJ, Sawitzki B. Interferon-γ: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 2006; 27:183–7.
25. Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collageninduced arthritis in IFN-γ receptor-deficient mice. J Immunol. 1997; 158:5507–13.
26. Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, et al. High susceptibility to collageninduced arthritis in mice lacking IFN-γ receptors. J Immunol. 1997; 158:5501–6.
27. Chu CQ, Song Z, Mayton L, Wu B, Wooley PH. IFN-γ deficient C57BL/6 (H-2b) mice develop collageninduced arthritis with predominant usage of T cell receptor Vβ6 and Vβ8 in arthritic joints. Ann Rheum Dis. 2003; 62:983–90.
28. Guedez YB, Whittington KB, Clayton JL, Joosten LA, van de Loo FA, van den Berg WB, et al. Genetic ablation of interferon-γ upregulates interleukin-1 expression and enables the elicitation of collageninduced arthritis in a nonsusceptible mouse strain. Arthritis Rheum. 2001; 44:2413–24.

29. Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, et al. TNF-α receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology. 2002; 42:93–106.
30. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001; 344:907–16.

31. Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996; 334:1717–25.

32. Taylor PC, Peters AM, Paleolog E, Chapman PT, Elliott MJ, McCloskey R, et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000; 43:38–47.
33. Horai R, Nakajima A, Habiro K, Kotani M, Nakae S, Matsuki T, et al. TNF-α is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. J Clin Invest. 2004; 114:1603–11.

34. Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003; 100:5986–90.

35. Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992; 256:105–7.

37. Nossal GJ, Pike BL, Battye FL. Mechanisms of clonal abortion tolerogenesis. II. Clonal behaviour of immature B cells following exposure to anti-mu chain antibody. Immunology. 1979; 37:203–15.
38. Boyd AW, Schrader JW. 1981. The regulation of growth and differentiation of a murine B cell lymphoma. II. The inhibition of WEHI 231 by anti-immunoglobulin antibodies. J Immunol. 1981; 126:2466–9.
39. Tsubata T. Apotosis of mature B cells. Int Rev Immunol. 1999; 18:347–65.
Go to : 
 | Fig. 1.Expression of costimulatory molecule (CD40, CD80, CD86, MHCII, PD-1, and PD-L1) was measured in splenic CD19+ B cells following stimulation (24 h) with LPS, anti-IgM, agonist CD40 mAb, or anti-IgM plus CD40 mAb as described in Materials and Methods. (A) Numbers indicate the percentage of positive cells estimated from the histograms. (B) Mean and standard deviation of mean fluorescence intensity of PD-L1expression in CD19+ B cells. Three independent experiments were performed. ∗p <0.01 vs. anti-IgM stimulation control and ∗∗p <0.05. |
 | Fig. 2.PD-L1 expression in CD19+ B cells stimulated with LPS, anti-IgM, CD40 mAb, or anti-IgM plus CD40 mAb for 3, 6, 9, 12, or 24 h. (A) Histograms represent staining of PD-L1; filled histograms indicate isotype control, dotted line histograms indicate untreated control, and black line histograms indicate each stimulation control. Numbers indicate mean fluorescence intensity (MFI). (B) Mean and standard deviation of PD-L1 MFI. Four independent experiments were performed.†p<0.05,††p<0.01 vs. anti-IgM stimulation control and ∗∗p<0.01 vs. CD40 mAb stimulation control. |
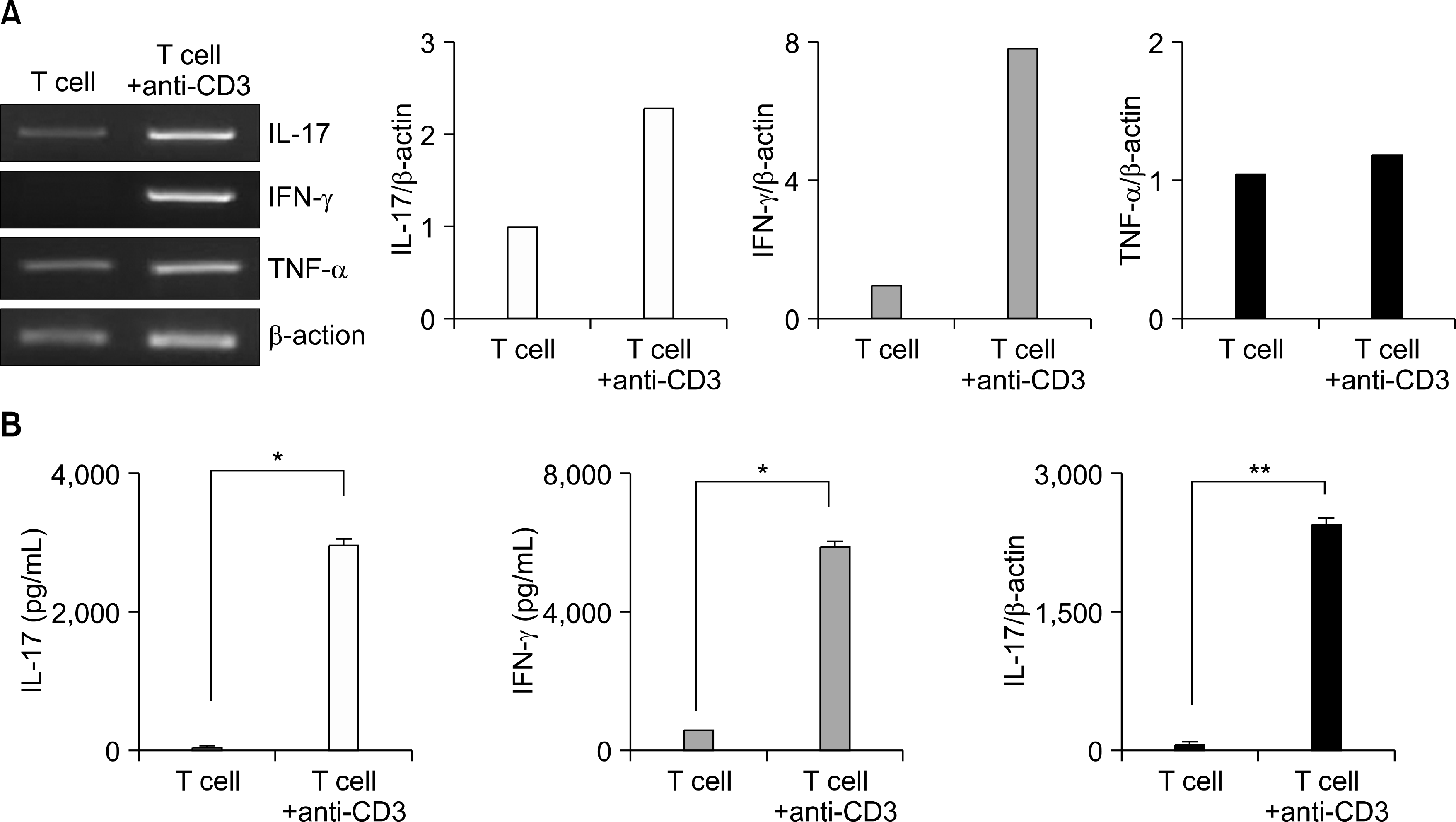 | Fig. 3.Increased expression of IL-17, IFN-γ and TNF-α on the CD3mAb stimulated CD4+ T cells. (A) IL-17, IFN-γ, and TNF-α mRNA expression induced by CD3mAb in CD4+ T cells. Cells were cultured in CD3mAb coated 24-well plates at a concentration of 5×105/well for 72 h. The expression of IL-17, IFN-γ, and TNF-α mRNA was evaluated by RT-PCR. Optical densities were normalized to the band for β-actin. (B) The concentrations of IL-17, IFN-γ and TNF-α in the culture supernatant were measured by sandwich ELISA analysis. The data represent the means from three independent. ∗p<0.05, ∗∗p<0.01 vs. nil. |
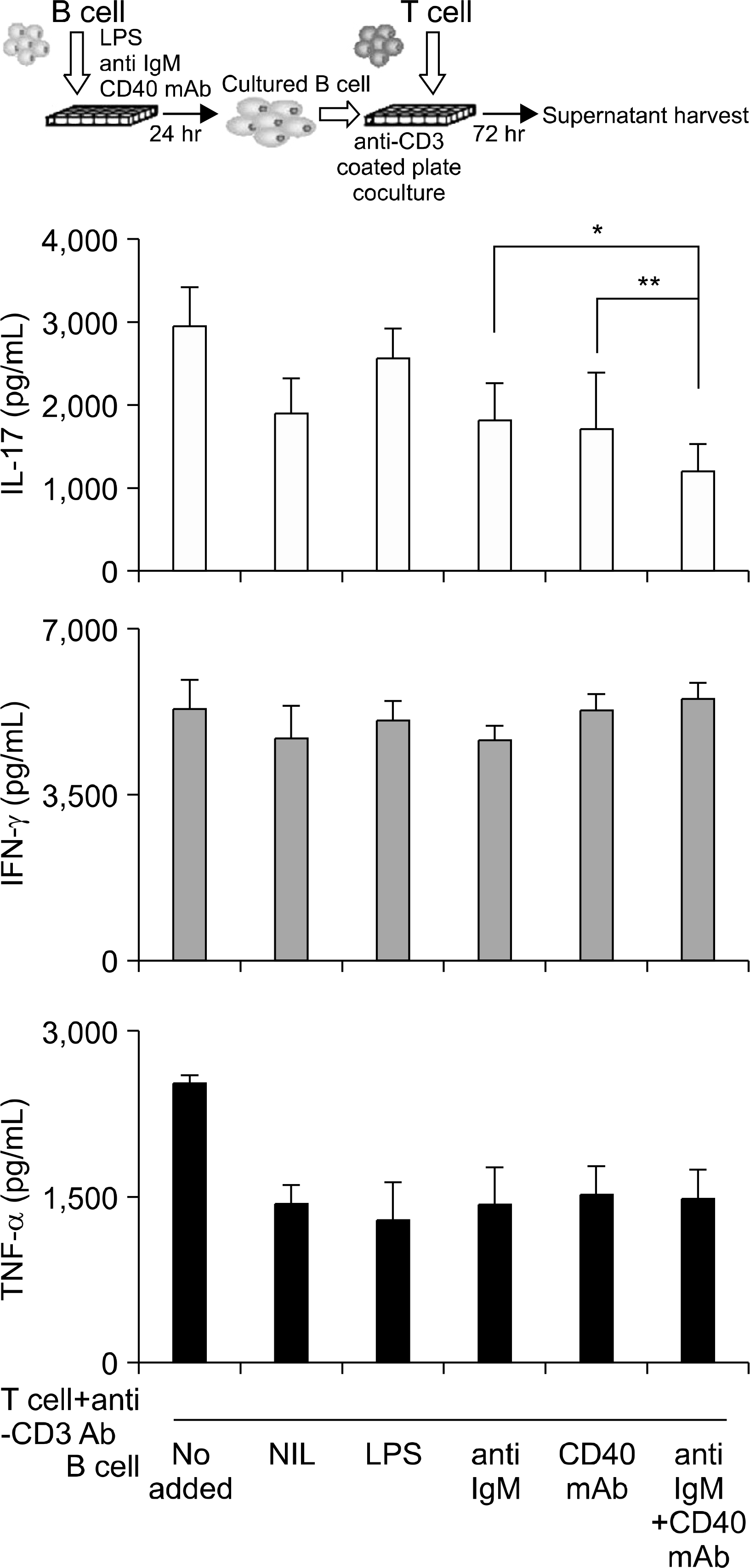 | Fig. 4.Cytokines production in the supernatants from CD4+ T cells stimulated with CD3 mAb cocultured with CD19+ B cells that had been stimulated with LPS, anti-IgM, CD40 mAb and anti-IgM plus CD40 mAb. IL-17, IFN-γ and TNF-α expression were measured by ELISA. Three independent experiments were performed. ∗p<0.01 vs. anti-IgM stimulation control and ∗∗p <0.01 vs. CD40 mAb stimulation control. |
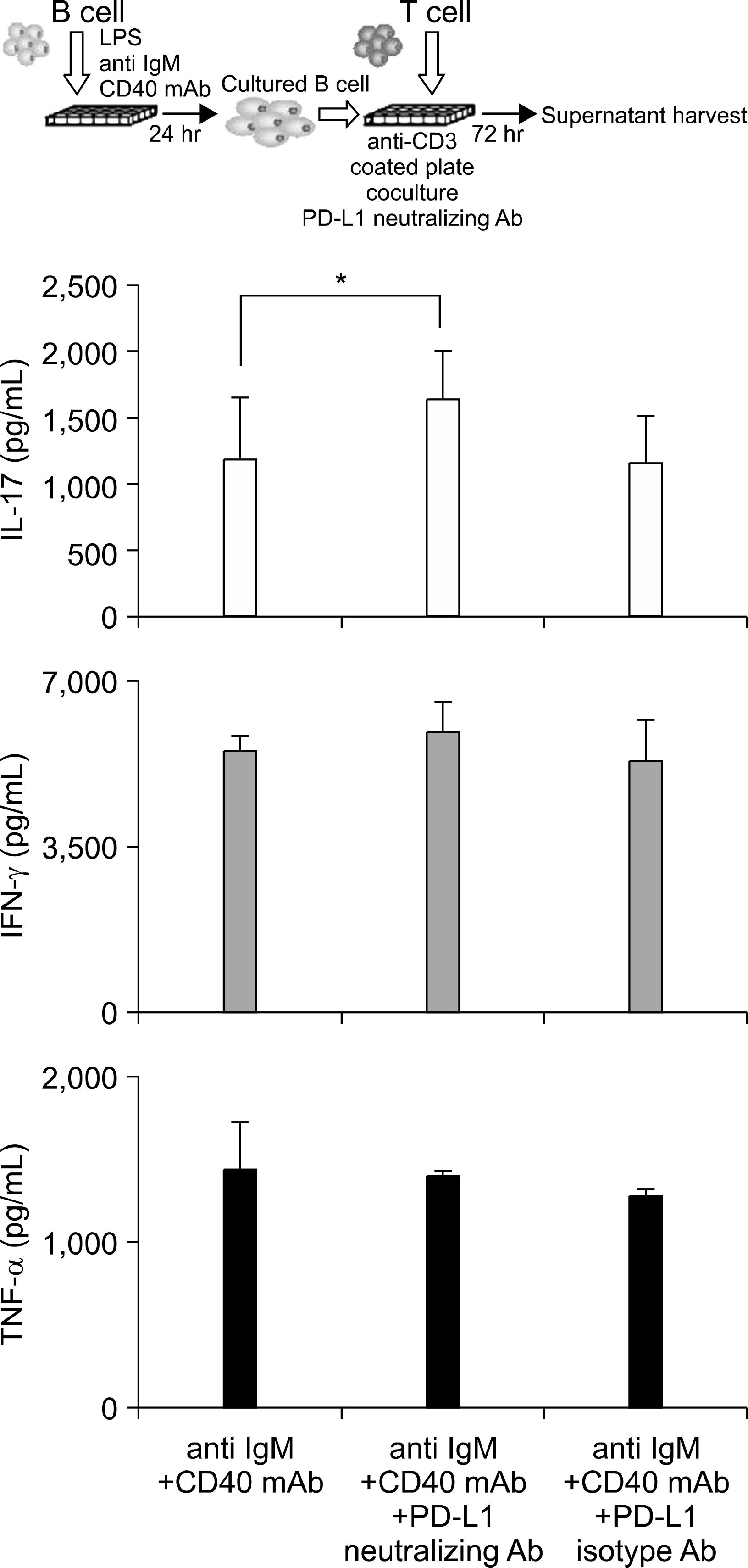 | Fig. 5.Cytokines production in the supernatants from CD4+ T cells stimulated with CD3 mAb cocultured with CD19+ B cells that had been stimulated with anti-IgM plus CD40 mAb and anti-IgM plus CD40 mAb plus PD-L1-blocking Ab. IL-17, IFN-γ and TNF-α expression were measured by ELISA. Three independent experiments were performed. ∗p<0.05, vs. anti-IgM plus CD40 mAb stimulation control. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download