Abstract
Objective
The acidic saline animal model of pain has been suggested to mimic fibromyalgia (FM). In this model, repeated intramuscular (IM) injections of acidic saline produce a widespread hyperalgesia that persists without evidence of significant peripheral tissue damage or inflammation, and is believed to be centrally maintained. We examined the changes of pain-related neurotransmitters in specific brain regions of this model after deep-sea water (DSW) drinking.
Methods
Rats were injected with 100μL of acidic saline (pH 4.0) at days 0 and 5 into the left gastrocnemius muscle. Control rats received identical injections of physiological saline (pH 7.2) on the same schedule. Two acidic saline rats were given DSW from 1 week following the last IM injection to sacrifice. All rats were sacrificed on day 20. All regions of interest were examined for the changes of pain-related neurotransmitters with immunohistochemistry.
Results
Preliminary results showed that compared to controls, acid injected rats demonstrated strong expression of serotonin in red and raphe nucleus. Acid injected rats showedsignificant reductions of the serotonin expression in red and raphe nucleus after DSW drinking.
Conclusion
IM acid injections increased the expression of serotonin in red and raphe nucleus of rats. The overwhelming reduction of serotonin expression in the nuclei after DSW drinking suggests DSW might be helpful for pain and anxiety. These preliminary data support the validity of acidic saline treatment as a model of FM, and provide a foundation for future analyses of specific brain regions that contribute to this syndrome.
Go to : 
References
1. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990; 33:160–72.
2. Okifuji A, Turk DC, Marcus DA. Comparison of generalized and localized hyperalgesia in patients with recurrent headache and fibromyalgia. Psychosom Med. 1999; 61:771–80.

3. Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001; 91:165–75.

4. Staud R. The neurobiology of chronic musculoskeletal pain. Wallace DJ, Clauw DJ, editors. Fibromyalgia & other central pain syndromes. 1st ed.p.p. 45–62. Philadelphia: Lippincott Williams & Wilkins;2005.
5. Schwarz MJ, Spath M, Muller-Bardorff H, Pongratz DE, Bondy B, Ackenheil M. Relationship of substance P, 5-hydroxyindole acetic acid and tryptophan in serum of fibromyalgia patients. Neurosci Lett. 1999; 259:196–8.

6. Offenbaecher M, Bondy B, de-Jonge S, Glatzeder K, Krger M, Schoeps P, et al. Possible association of fibromyalgia with a polymorphism in the serotonin transporter gene regulatory region. Arthritis Rheum. 1999; 42:2482–8.

7. Russell IJ. Advances in fibromyalgia: possible role for central neurochemicals. Am J Med Sci. 1998; 315:377–84.

8. Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996; 66:2621–4.

9. Moldofsky H. Rheumatic pain modulation syndrome: the interrelationships between sleep, central nervous system serotonin, and pain. Adv Neurol. 1982; 33:51–7.
10. Harris RE, Clauw DJ. How do we know that the pain in fibromyalgia is "real"? Curr Pain Headache Rep. 2006; 10:403–7.

11. Guedj E, Taieb D, Cammilleri S, Lussato D, de Laforte C, Niboyet J, et al. (99m)Tc-ECD brain perfusion SPECT in hyperalgesic fibromyalgia. Eur J Nucl Med Mol I. 2007; 34:130–4.

12. Kwiatek R, Barnden L, Tedman R, Jarrett R, Chew J, Rowe C, et al. Regional cerebral blood flow in fibromyalgia: single-photon-emission computed tomography evidence of reduction in the pontine tegmentum and thalami. Arthritis Rheum. 2000; 43:2823–33.

13. Mountz JM, Bradley LA, Modell JG, Alexander RW, Triana-Alexander M, Aaron LA, et al. Fibromyalgia in women. Abnormalities of regional cerebral blood flow in the thalamus and the caudate nucleus are associated with low pain threshold levels. Arthritis Rheum. 1995; 38:926–38.
14. Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001; 24:37–46.

15. Harte SE, Kim SH, Clauw DJ, Morrow TJ. Altered regional cerebral blood flow at rest in an animal model of fibromyalgia. Arthritis Rheum. 2007; 56(Suppl):): S92.
16. Hataguchi Y, Tai H, Nakajima H, Kimata H. Drinking deep-sea water restores mineral imbalance in atopic eczema/dermatitis syndrome. Eur J Clin Nutr. 2005; 59:1093–6.

17. Yoshikawa S, Hamada A, Gue T, Yokota J, Yamamoto S, Kusunose M, et al. Pharmacological activity of deep-sea water: examination of hyperlipemia prevention and medical treatment effect. Biol Pharm Bull. 2003; 26:1552–9.
18. Miyamura M, Yoshioka S, Hamada A, Takuma D, Yokota J, Kusunose M, et al. Difference between deep seawater and surface seawater in the preventive effect of atherosclerosis. Biol Pharm Bull. 2004; 27:1784–7.

19. Katsuda SI, Yasukawa T, Nakagawa K, Miyake M, Yamasaki M, Katahira K, et al. Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol Pharm Bull. 2008; 31:38–44.

21. Eccles JC, Scheid P. Taborikova H. Responses of red nucleus neurones to cutaneous afferent inputs. Brain Res. 1973; 53:440–4.
22. Larsen KD, Yumiya H. The red nucleus of the monkey. Topographical localization of somatosensory input and motor output. Exp Brain Res. 1980; 40:393–404.
23. Padel Y, Sybirska E, Bourbannais D, Vinay L. Electrophysiological identification of a somaesthetic pathway of the red nucleus. Behav Brain Res. 1988; 8:139–51.
24. Vinay L, Padel Y. Spatiotemporal organization of the somaesthetic projections in the red nucleus transmitted through the spino-rubral pathway in the cat. Exp Brain Res. 1990; 79:412–26.

25. Nishioka S, Nakahama H. Peripheral somatic activation of neurons in the cat red nucleus. J Neurophysiol. 1973; 36:296–307.

26. Prado WA, Raghubir R, Roberts MHT. Long duration antinociception induced by red nucleus stimulation in the rat. Pain. 1984; 2(Suppl):329S.

27. Matsumoto RR, Walker JM. Inhibition of rubral neurons by noxious and non-noxious pressure. Brain Res. 1991; 556:78–84.

28. Palkovitz M, Brownstein M, Saavedra JM. Serotonin content of the brainstem nuclei in the rat. Brain Res. 1974; 80:237–49.
29. Steinbusch HWM. Serotonin-immunoreactive neurons and their projection in the CNS. In: Björklund A, Hokfelt T, Kuhar MJ, eds. Handbook of chemical neuroanatomy. Vol III. p.68–125. Amsterdam, Elsevier,. 1984.
30. Pierce ET, Foote WE, Hobson JA. The efferent connection of the nucleus raphe dorsalis. Brain Res. 1976; 107:137–44.
31. Hobson JA, Lydic R, Baghdoyan HA. Evolving concepts of sleep cycle generation: from brain centers to neuronal populations. Behav Brain Sci. 1986; 9:371–448.

32. Jouvet M. Biogenic amines and states of sleep. Science. 1969; 163:32–41.
33. Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984; 7:309–38.

34. Kahn RS, van Praag HM, Wetzler S, Asnis GM, Barr G. Serotonin and anxiety revisited. Biol Psychiatry. 1988; 23:189–208.

35. Miller LE, van Kan PL, Sinkjaer T, Andersen T, Harris GD, Houk JC. Correlation of primate red nucleus discharge with muscle activity during free-form arm movements. J Physiol. 1993; 469:13–43.

36. Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Rev. 2002; 39:54–80.

37. Graeff FG, Guimaress FS, De Andrade TGCS, Deakin JFW. Role of S-NT in stress, anxiety and depression. Pharmacol Biochem Behav. 1996; 54:129–41.
38. Matos FF, Urban C, Vocca FD. Serotonin release in the dorsal raphe and ventral hippocampus: raphe control of somatodendritis and terminal 5-HT release. J Neural Transm. 1996; 103:173–90.
Go to : 
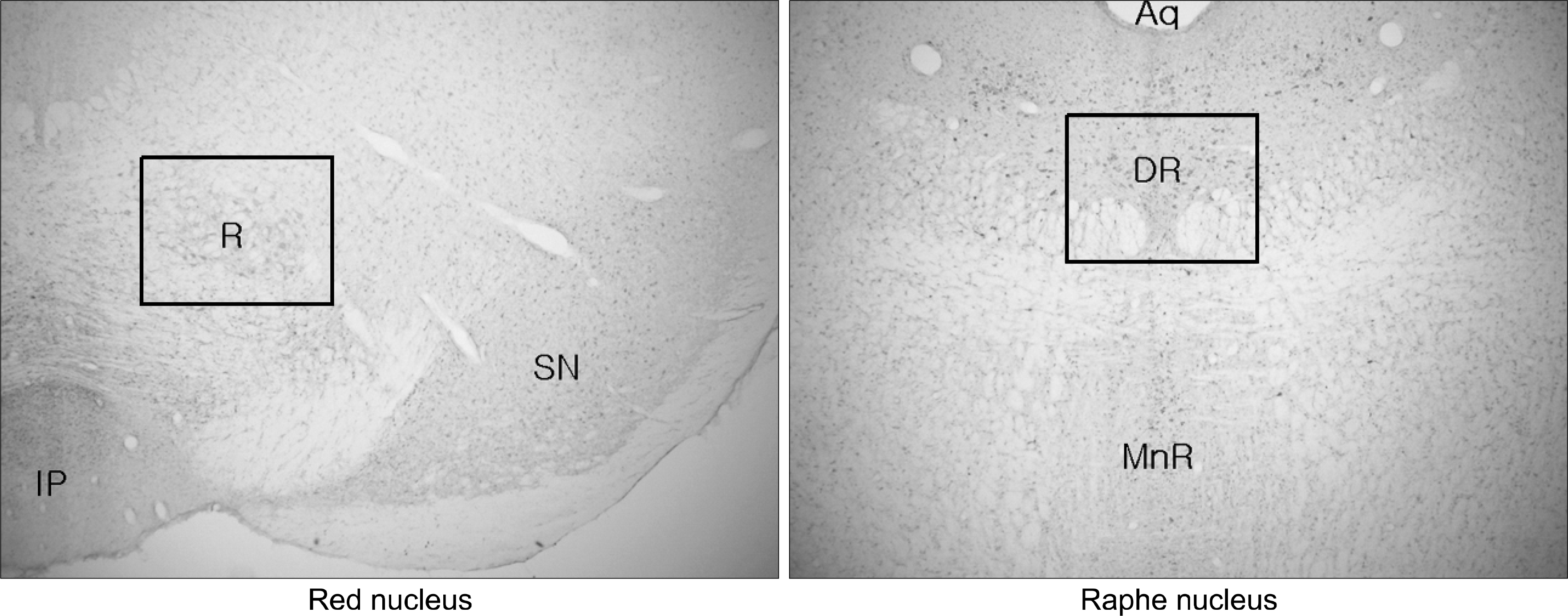 | Fig. 1.Immunohistochemistry stain of serotonin from brain of rats. Rectangle area will be magnified in next figures. Aq: aqueduct, DR: dorsal raphe nucleus, IP: interpeduncular nucleus, MnR: median raphe nucleus, R: red nucleus, SN: substantia nigra. |
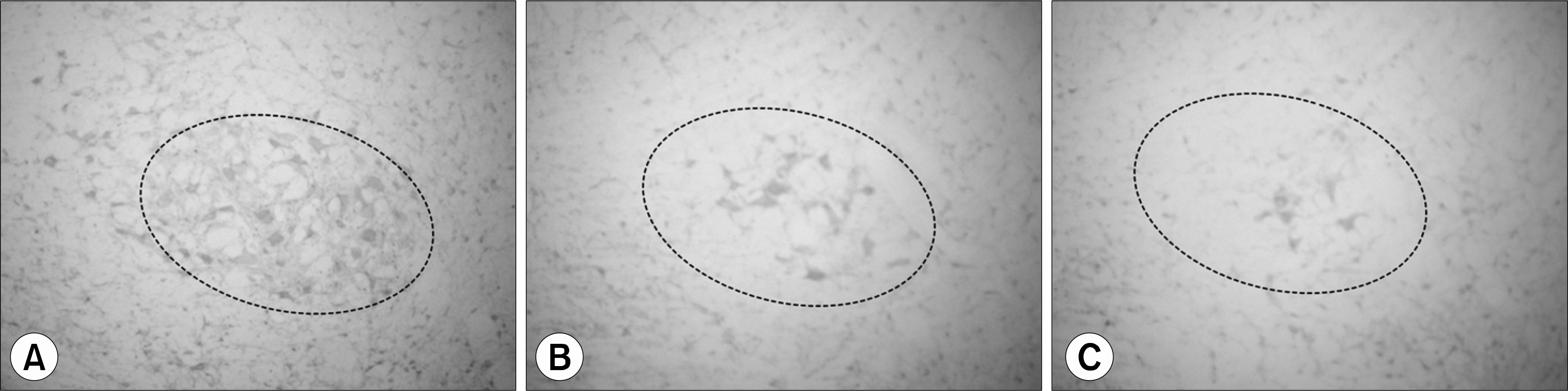 | Fig. 2.Immunohistochemistry stain of serotonin from red nucleus of rats (×100). (A) Water after pH4 injection, (B) DSW pH4 injection, (C) water after pH7 injection, DSW: deep-sea water. |
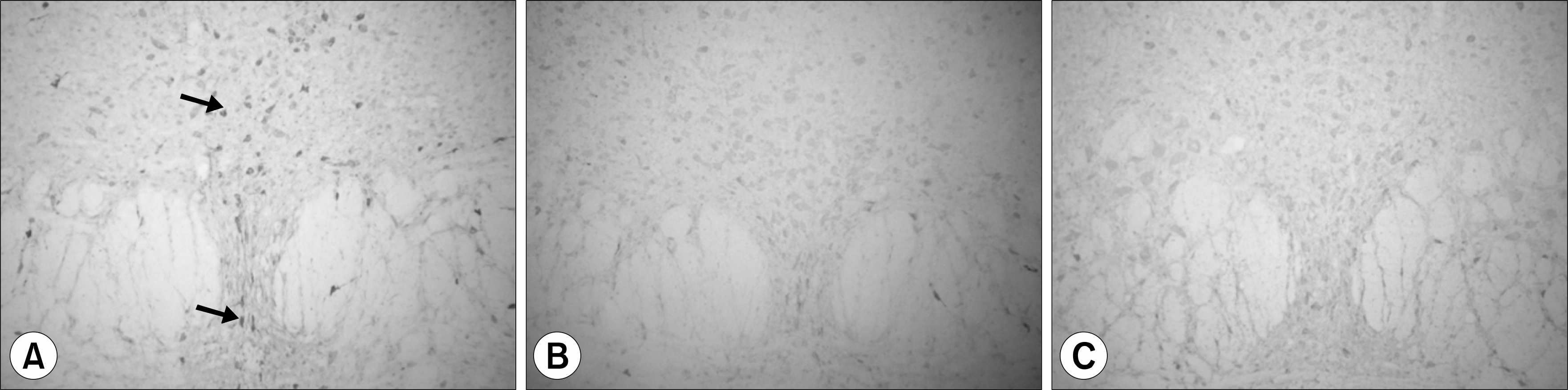 | Fig. 3.Immunohistochemistry stain of serotonin from raphe nucleus of rats. (×100) (A) water after pH4 injection, (B) DSW after pH4 injection, (C) water after pH7 injection, DSW: deep-sea water. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download