Abstract
Osteoblastic differentiations of mesenchymal stem cells are controlled by a number of intracellular signal transduction systems and transcription factors. These systems and factors interacts with and cross-talks to one another to complete the process of bone formation. Discovery of these systems has been enabled by gene-targeting techniques. In this brief review, the signal transduction pathways and transcription factors of importance to osteoblastic differentiation are introduced.
References
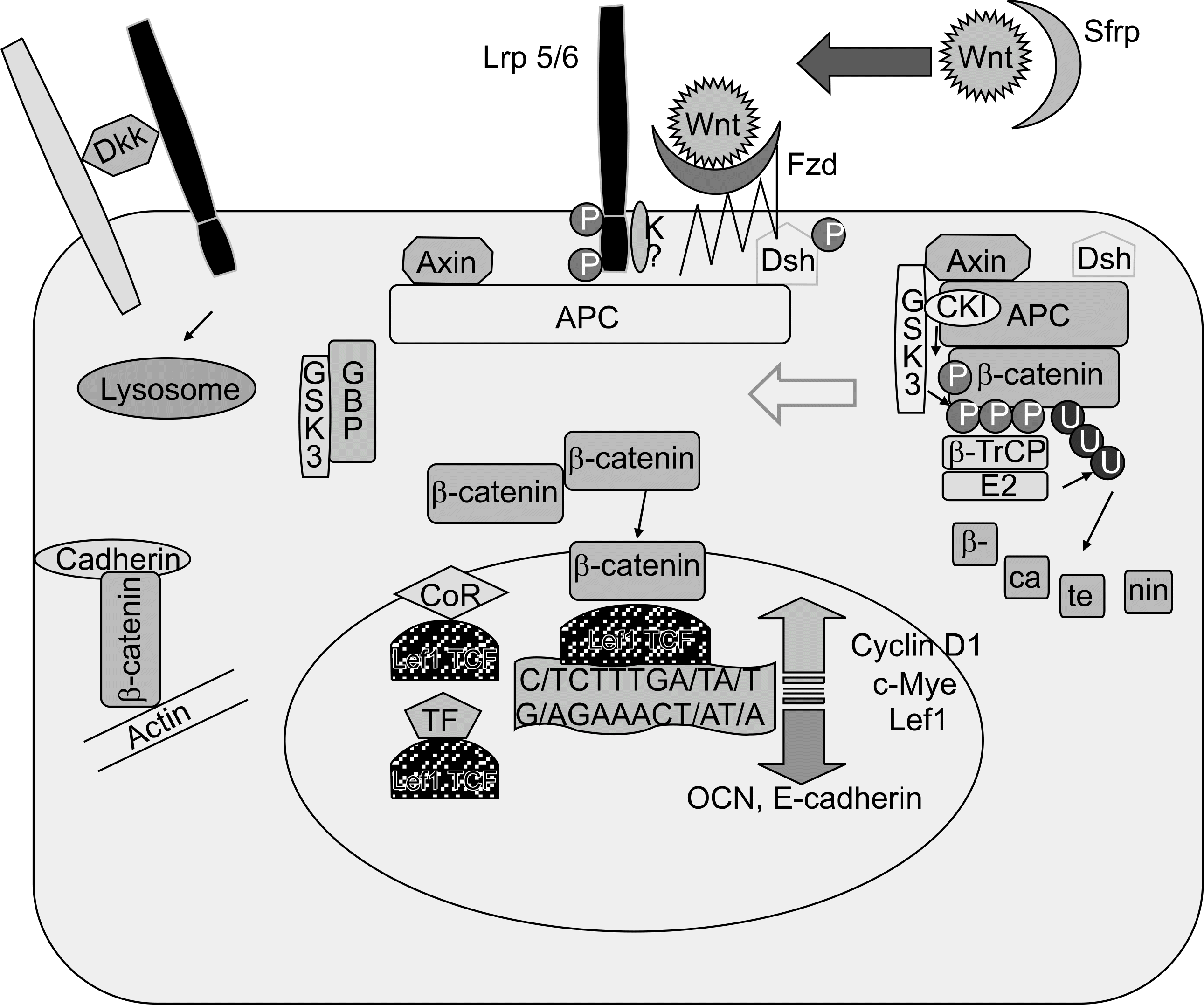
1. Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dec Cell. 2003; 5:367–77.
2. Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004; 341:19–39.

3. Rawadi G, Roman-Roman S. Wnt signaling pathway: a new target for the treatment of osteoporosis. Expert Opin Ther Targets. 2005; 9:1063–77.
4. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004; 20:781–810.

5. Moon RT, Kohn AD, De Ferrari GV, Kaykas A. Wnt and beta-catenin signaling: diseases and therapies. Nat Rev Genet. 2004; 5:691–701.
6. Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005; 280:26770–5.
7. Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signaling. Nature. 2002; 417:664–7.
8. Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003; 115:151–62.

9. Yamaguchi TP, Bradley PA, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999; 126:1211–23.

10. Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003; 130:1003–15.

11. Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001; 107:513–23.
12. Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signalin. Mol Cell Biol. 2005; 25:4946–55.
13. Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002; 346:1513–21.

14. Van Wesenbeek L, Cleiren LE, Gram J, Beals RK, Benichou O, Scopelliti D, et al. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet. 2003; 72:763–71.
15. Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, et al. Cbfal-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002; 157:303–14.
16. Jackson A, Vayssiere B, Garcia T, Newell W, Baron R, Roman-Roman S, et al. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005; 36:585–98.

17. Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, et al. Canonical Wnt dignaling in differentiated osteoblasts controls osteoblast differentiation. Dev Cell. 2005; 8:751–64.
18. Henriksen K, Gram J, Hoegh-Andersen , Jemtland R, Ueland T, Dziegkel MH, et al. Osteoblast from patients with autosomal dominant osteopetrosis type I caused by a T253I mutation in low-density lipoprotein receptor-related protein 5 are normal in vitro, but have decreased resorption capacity in vivo. Am J Pathol. 2005; 167:1341–8.
19. Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005; 8:739–50.
20. Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005; 8:727–38.
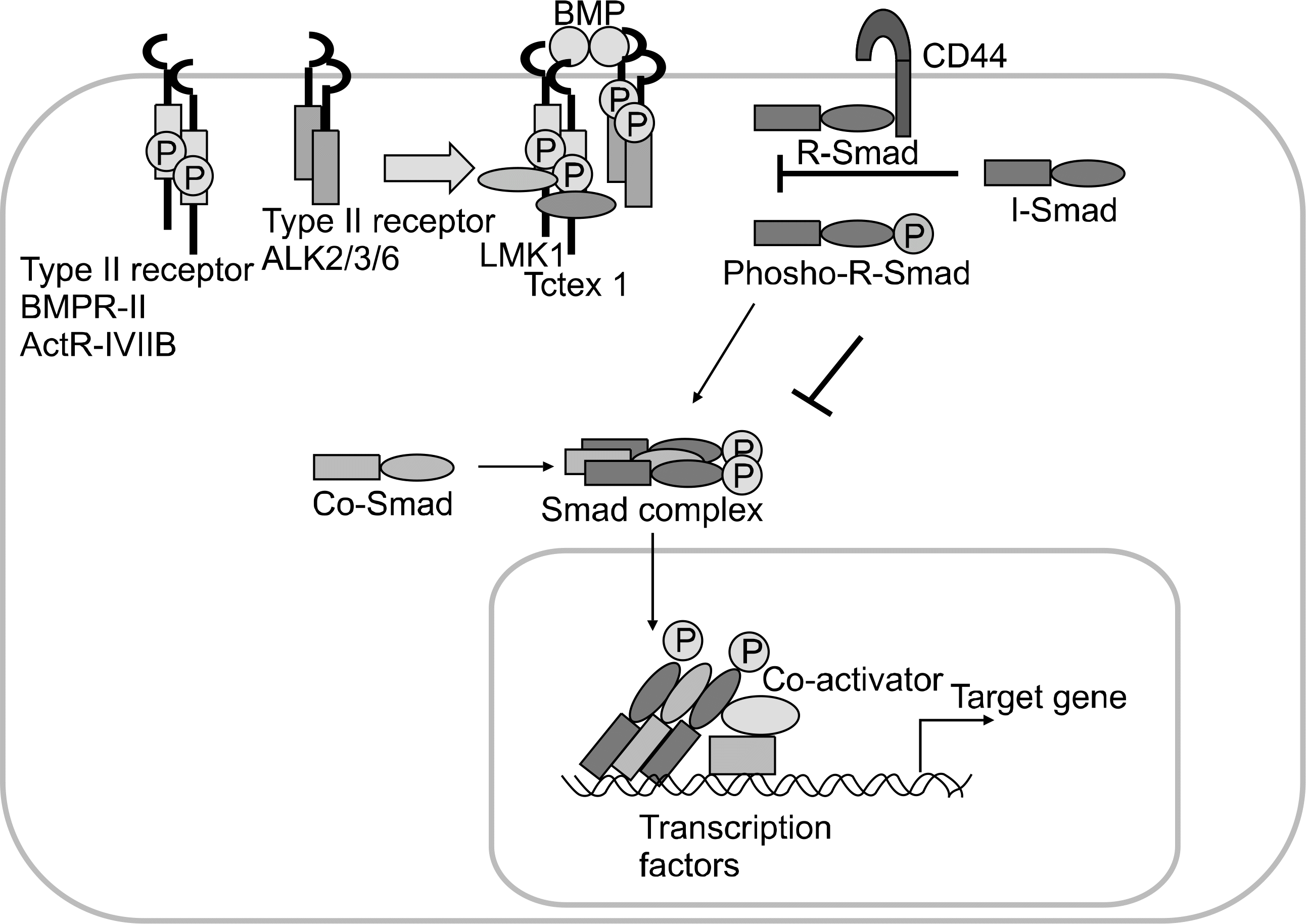
21. Kluppel M, Hoodless PA, Wrana JL, Attisano L. Frontiers in molecular biology: protein kinase funtions. 1st ed. p.303–40. Oxford, Oxford University Press,. 2000.
22. Herpin A, Lelong C, Favrel P. Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol. 2004; 28:461–85.
23. Massague J, Seoane J, Wotton D. Smad transcrption factors. Genes Dev. 2005; 19:2783–810.
24. Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling crosstalk. Cytokine Growth Factor Rev. 2005; 16:251–63.

25. Shi Y, Massague J. Mechanism of TGF-B signaling from cell membrane to the nucleus. Cell. 2003; 113:685–700.
26. Heldin CH, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1998; 390:465–71.
27. Deynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor supperssion and cancer progression. Nat Genet. 2001; 29:117–29.
28. Ogata T, Wozney JM, Benezra R, Noda M. Bone morphogenetic protein 2 transiently enhances expression of a gene. Id (inhibitor of differentiation), encoding a helix-loop-helix molecule in osteoblast like cells. Proc Natl Acad Sci USA. 1993; 90:9219–22.
31. Komori T. Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem. 2005; 95:445–53.

32. Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974; 249:123–7.

33. Eswarakumar VP, Las I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor. 2005; 16:139–49.

34. Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991; 64:841–8.

35. Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991; 252:1705–8.

36. Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P. Heparin is requirement for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992; 12:240–7.
37. Spivak-Kroizman T, Lemmon MA, Dikie I, Ladbury JE, Pinchasi D, Huang J, et al. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell. 1994; 79:1015–24.

38. Lin X, Buff EM, Perrimon N, Michelson AM. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 1999; 126:3715–23.

39. Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996; 84:911–21.

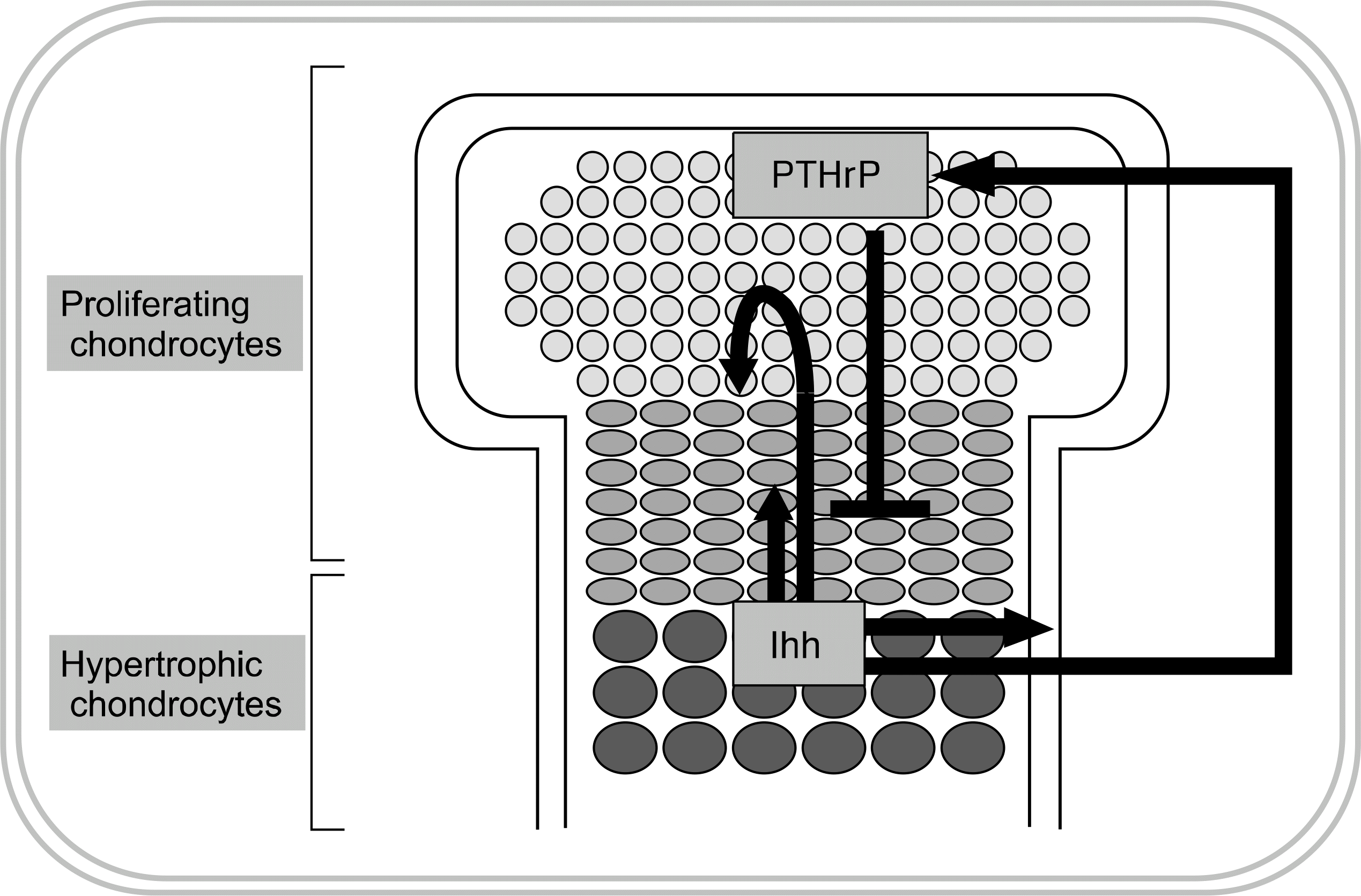
40. Lick PL, Jane M. Indian hedgehog: its roles and regulation in endochondral bone development. J Cell Biochem. 2005; 96:1163–73.
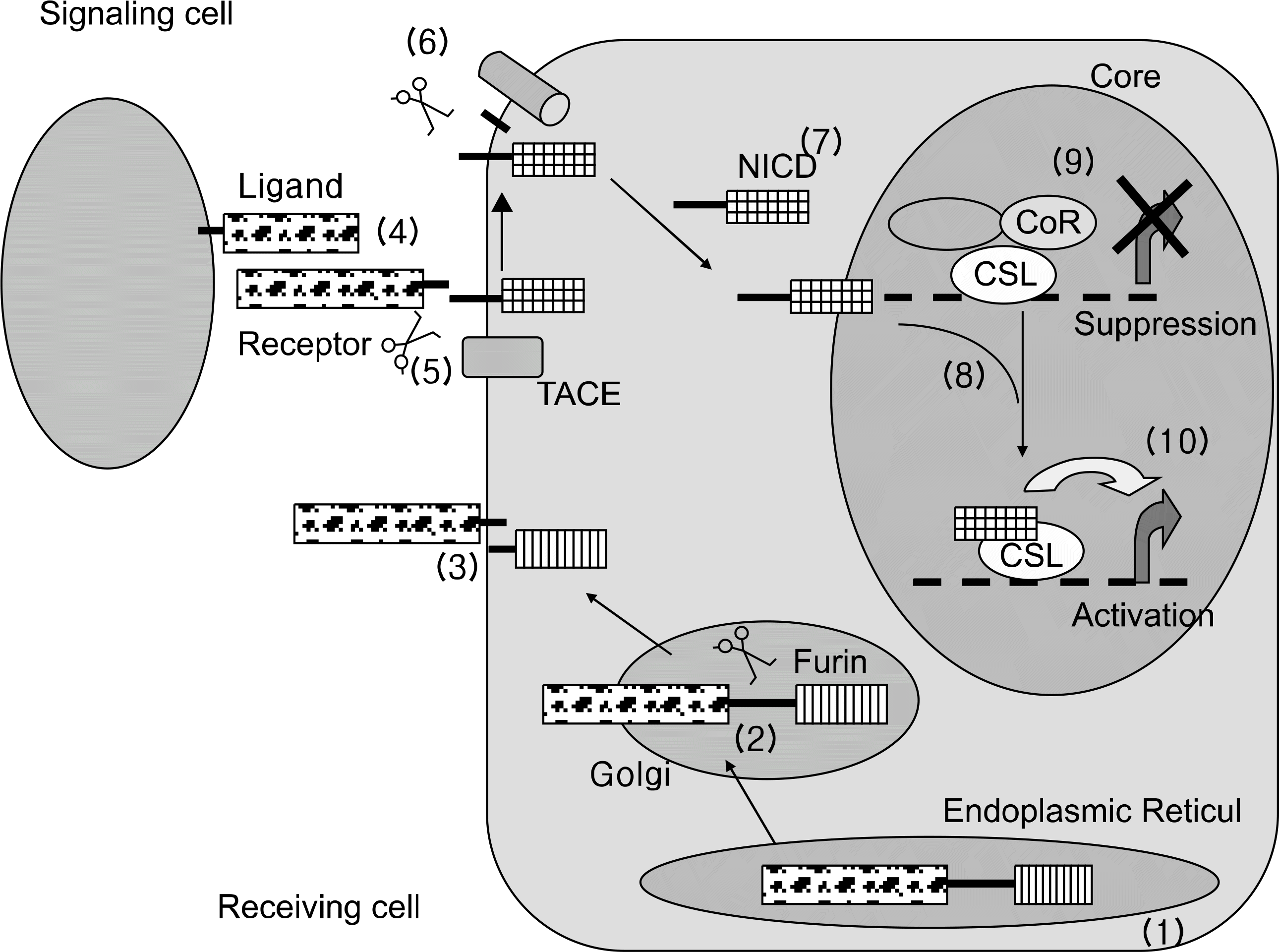
41. Harper JA, Yuan JS, Tan JB, Visan I, Guidos CJ. Notch signaling in development and disease. Clin Genet. 2003; 64:461–72.

43. Michael K, Jeffery LW. Turning it up a Notch: crosstalk between TGFβ and Notch signaling. BioAssays. 2005; 27:115–8.
42. Baron M. An overview of the Notch signaling pathway. Semin Cell Dev Biol. 2003; 14:113–9.
44. Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003; 425:841–6.

45. Watanabe N, Tezuka Y, Matsuno K, Miyatani S, Morimura N, Yasuda M, et al. Suppression of differentiation and proliferation of early chondrogenic cells by Notch. J Bone Miner Metab. 2003; 21:344–52.

46. Nobta M, Tsukazaki T, Shibata Y, Xin C, Moriishi T, Sakano S, et al. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem. 2005; 280:15842–8.

47. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfal results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997; 89:755–64.
48. Kobayashi H, Gao Y, Ueta C, Yamaguchi A, Komori T. Multilineage differentiation of Cbfaldeficient calvarial cells in vitro. Biochem Biophys Res Commun. 2000; 273:630–6.
49. Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006; 99:1233–9.

50. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002; 108:17–29.

51. Nishio Y, Dong Y, Paris M, O'Keefe RJ, Schwarz EM, Drissi H. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene. 2006; 372:62–70.

52. Kim YJ, Kim HN, Park EK, Lee BH, Ryoo HM, Kim SY, et al. The bone-related Zn finger transcription factor Osterix promotes proliferation of mesenchymal cells. Gene. 2006; 366:145–51.

53. Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, et al. Cell growth regulatory role of Runx2 during proliferative expansion of pre-osteo-blasts. Cancer Res. 2003; 63:5357–62.
54. Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005; 202:1261–9.

55. Dodig M, Tadic T, Kronenberg MS, Dacic S, Liu YH, Maxson R, et al. Ectopic Msx2 overexpression inhibits and Msx2 antisense stimulates calvarial osteoblast differentiation. Dev Biol. 1999; 209:298–307.
56. Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun. 2003; 309:689–94.

57. Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, et al. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003; 278:34387–94.
58. Hassan MQ, Javed A, Morasso MI, Karlin J, Montecino M, van Wijnen AJ, et al. Dlx3 transcriptional regulation of osteoblast differentiation: temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol Cell Biol. 2004; 24:9248–61.

59. Ichida F, Nishimura R, Hata K, Matsubara T, Ikeda F, Hisada K, et al. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem. 2004; 279:34015–22.

60. Yoshizawa T, Takizawa F, Iizawa F, Ishibashi O, Kawashima H, Matsuda A, et al. Homeobox protein MSX2 acts as a molecular defense mechanism for preventing ossification in ligament fibroblasts. Mol Cell Biol. 2004; 24:3460–72.

61. Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003; 278:45969–77.

62. Tadic T, Dodig M, Erceg I, Marijanovic I, Mina M, Kalajzic Z, et al. Overexpression of Dlx5 in chicken calvarial cells accelerates osteoblastic differentiation. J Bone Miner Res. 2002; 17:1008–14.

63. Lee MH, Kim YJ, Yoon WJ, Kim JI, Kim BG, Hwang YS, et al. Dlx5 specifically regulates Runx2-II expression by binding to homeodomain response elements in the Runx2 distal promoter. J Biol Chem. 2005; 280:35579–87.
64. Xiao G, Jiang D, Ge C, Zhao Z, Lai Y, Boules H, et al. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J Biol Chem. 2005; 280:30689–96.

65. Gutierrez S, Javed A, Tennant D, van Rees M, Mon tecino M, Stein GS, et al. CCAAT/enhancer-binding proteins (C/EBP) b and d Activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J Biol Chem. 2002; 277:1316–23.
66. McCarthy TL, Ji C, Chen Y, Kim KK, Imagawa M, Ito Y, et al. Runt domain factor (Runx)-dependent effects on CCAAT/enhancer-binding protein delta expression and activity in osteoblasts. J Biol Chem. 2000; 275:21746–53.
67. Selvamurugan N, Chou WY, Pearman AT, Pulumati MR, Partridge NC. Parathyroid hormone regulates the rat collagenase-3 promoter in osteoblastic cells through the cooperative interaction of the activator protein-1 site and the runt domain binding sequence. J Biol Chem. 1998; 273:10647–57.

68. Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004; 117:387–98.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download