Abstract
Objecti
Although increased expression of receptor for advanced glycation end products (AGE) in osteoarthritis (OA) has been reported, little is known concerning the role of AGEs in the pathogenesis of OA. This study was undertaken to determine the effect of AGEs on the regulation of matrix metalloproteinase (MMP) expressions and activities in human OA chond- rocytes
Methods
OA chondrocytes were treated with increasing doses of AGE-bovine serum albumin (AGE-BSA). The expressions of MMPs were determined by both enzyme-linked immunosorbent assay (ELISA) and immunoblot analysis. The activities of MMPs were evaluated by both gelatin and casein zymography assays. In addition, electrophoretic mobility shift assay (EMSA) was employed to investigate the DNA binding activity of nuclear factor-kappa B (NF- κ B) by AGE-BSA treatment.
Results
The productions of MMP-1, -3, and-13 were significantly elevated by AGE-BSA in a dose dependent manner. The elevated activities of MMP-1, -3, and-13, and TNF-α by AGE-BSA were also observed. DNA binding activity of NF-B was markedly increased by AGE-BSA treatment implicating possible involvement of NF-^B mediated pathway in the AGE-BSA induced MMP-1, -3, and-13, and TNF-α productions in OA chondrocytes. Taken together, this study demonstrates the stimulatory effect of AGE-BSA on the productions of MMPs and TNF-α and suggests the possible involvement of NF-^B mediated pathway in OA chond- rocytes.
Go to : 
REFERENCES
1). DeGroot J., Verzijl N., Wenting-van Wijk MJ., Jacobs KM., Van El B., Van Roermund PM, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis is. Arthritis Rheum. 2004. 50:1207–15.
2). Felson DT., Lawrence RC., Dieppe PA., Hirsch R., Helmick CG., Jordan JM, et al. Osteoarthritis: new insight. Part 1: the disease and its risk factors. Ann intern Med. 2000. 133:635–46.
3). Swnolt L., Senolt L., Braun M., 이ejarova M., Forejtova S., Gatterova J, et al. Increased pentosidine, an advanced glycation end product, in serum and synovial fluid from patients with knee osteoarthritis and its relation with cartilage oligomeric matrix protein. Ann Rheum Dis. 2005. 64:886–90.
4). Muir H. The chondrocyte, architect of cartilage: bio-mechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays. 1995. 17:1039–48.

5). DeGroot J., Verzijl N., Wenting-Van Wijk MJ., Bank RA., Lafeber FP., Bijlsma JW, et al. Age-related decrease in susceptibility of human articular cartilage to matrix metalloproteinase-mediated degradation: the role of advanced glycation end products. Arthritis Rheum. 2001. 44:2562–71.

6). Vincenti MR., Brinckerhoff CE. Transcriptional regulation of collagenases (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res Ther. 2002. 4:157–64.

7). Martel-pelletier J., Welsch DJ., Pelletier JP. Metall-oproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001. 15:25–9.

8). Neuhold LA., Killar L., Zhao W., Sung ML., Warner L., Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Cli Invest. 2001. 107:35–44.

9). Flannery CR. Lark MW, Sandy JD. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992. 267:1008–14.
10). Verzijl N., DeGroot J., Thorpe SR., Bank RA., Shaw JN., Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000. 275:39027–31.

11). DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr ᄋpin Pharmacol. 2004. 4:301–5.

12). Verzijl N., DeGroot J., 이dehinkel E., Bank RA., Thorpe SR., Baynes JW, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000. 350:381–7.

13). Verzijl N., DeGroot J., Ben ZC., Brau-Benjamin ᄋ., Maroudas A., Bank RA, et al. Cross-linking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis, Arthritis Rheum. 2002. 46:114–23.
14). Veilleux NH., Yannas IV., Spector M. Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocytes in type I and II collagen-glycosaminoglycan matrices in vitro. Tissue Eng. 2004. 10:119–27.
15). Marlovits S., Hombauer M., Truppe M., Vecsei V., Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Joint Surg Br. 2004. 86:286–95.

16). Farboud B., Aotaki-Keen A., Miyata T., Hjelmeland LM., Handa JT. Development of a polyclonal antibody with broad epitope specificity for advanced glycation endproducts and localization of these epitopes in Bruch's membrane of the aging eye. Mol Vis. 1999. 5:11.
17). Cipollone F., Iezzi A., Fazia M., Zucchelli M., Pini B., Cuccurullo C, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003. 108:1070–7.
18). Federici M., Giustizieri ML., Scarponi C., Girolomoni G., Albanesi C. Impaired IFN-gamma-dependent inflammatory responses in human keratinocytes over-expressing the suppressor of cytokine signaling 1. J Immunol. 2002. 169:434–42.
19). Hofmann MA., Drury S., Fu C., Qu W., Taguchi A., Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/cal-granulin polypeptides. Cell. 1999. 97:889–901.
20). Yeh CH., Sturgis L., Haidacher J., Zhang XN., Sherwood SJ., Bjercke RJ, et al. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001. 50:1495–504.
21). Huttunen HJ., Fages C., Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999. 274:19919–24.
22). Huang JS., Guh JY., Chen HC., Hung WC., Lai YH., Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem. 2001. 81:102–13.

23). DeGroot J., Verzijl N., Budde M., Bijlsma JW., Lafeber FP., TeKoppele JM. Accumulation of advanced glycation end products decreases collagen turnover by bovine chondrocytes. Exp Cell Res. 2001. 266:303–10.

24). DeGroot J., Verzijl N., Jacobs KM., Budde M., Bank RA., Bijlsma JW, et al. Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthritis Cartilage. 2001. 9:720–6.

Go to : 
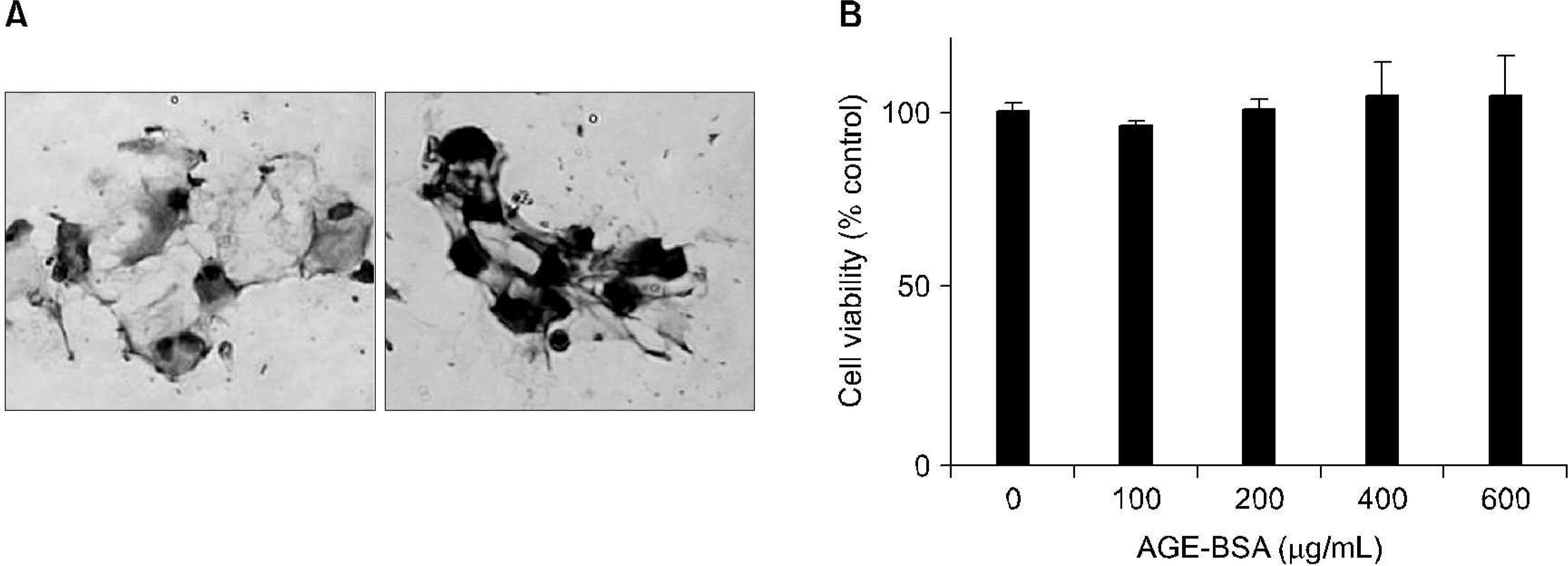 | Fig. 1.Immunocytochemical staining for type I and II collagen in monolayer-cultured osteoarthritis (OA) chondrocytes. Spherical or polygonal cells containing a single eccentric nucleus were observed. Type II (right panel), but not type I (left panel), collagen was detected in OA chondrocytes 7 days after primary culture (A). The effect of advanced glycation end product bovine serum albumin (AGE-BSA) on the viability of OA chondrocytes (B). |
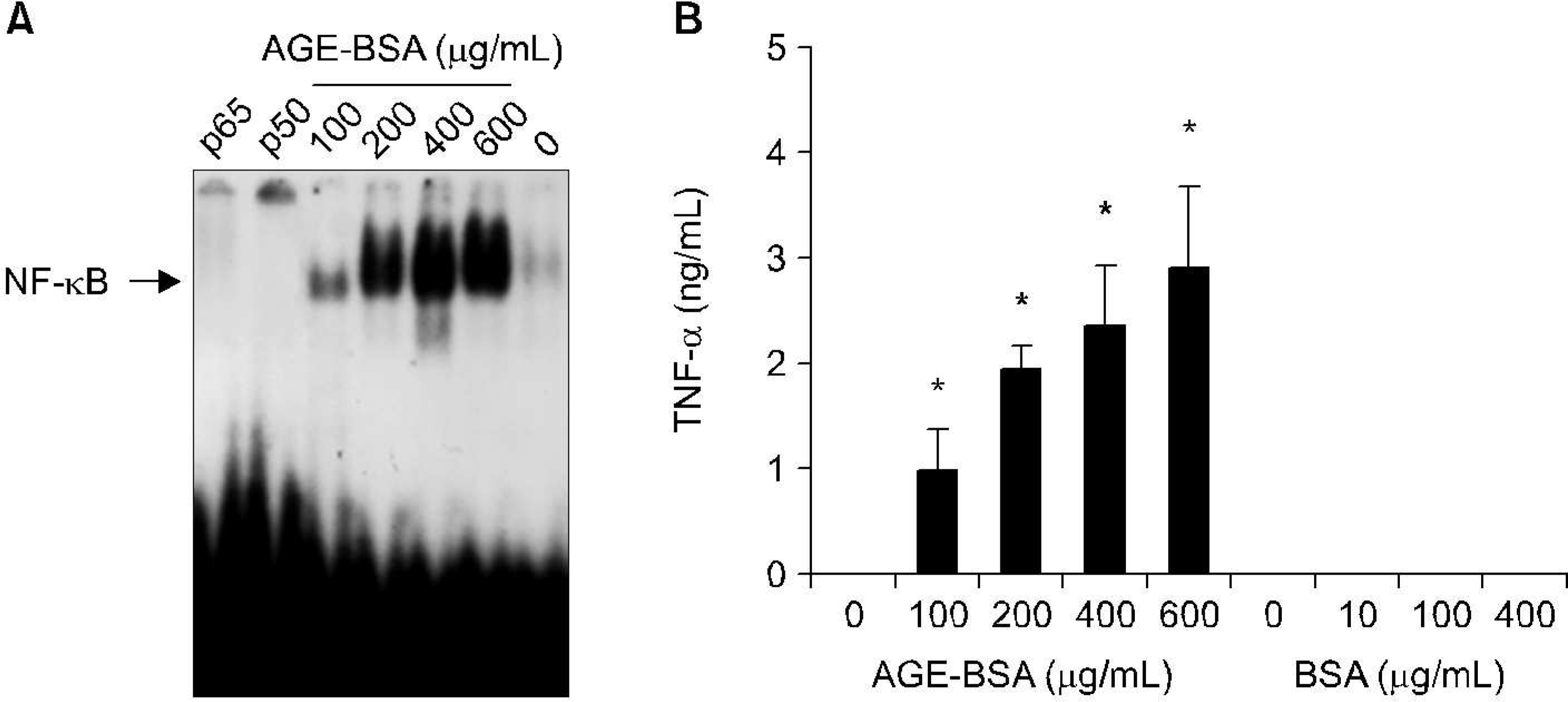 | Fig. 2.Induction of nuclear factor-kappa B (NF- kB) (A), and tumor necrosis factor- α (TNF- α) expression by advanced glycation end product bovine serum albumin (AGE-BSA) in human osteoarthritis (OA) chondrocytes (B). n=4, ∗p<0.05 compaired with none and AGE-BSA or BSA treatment. |
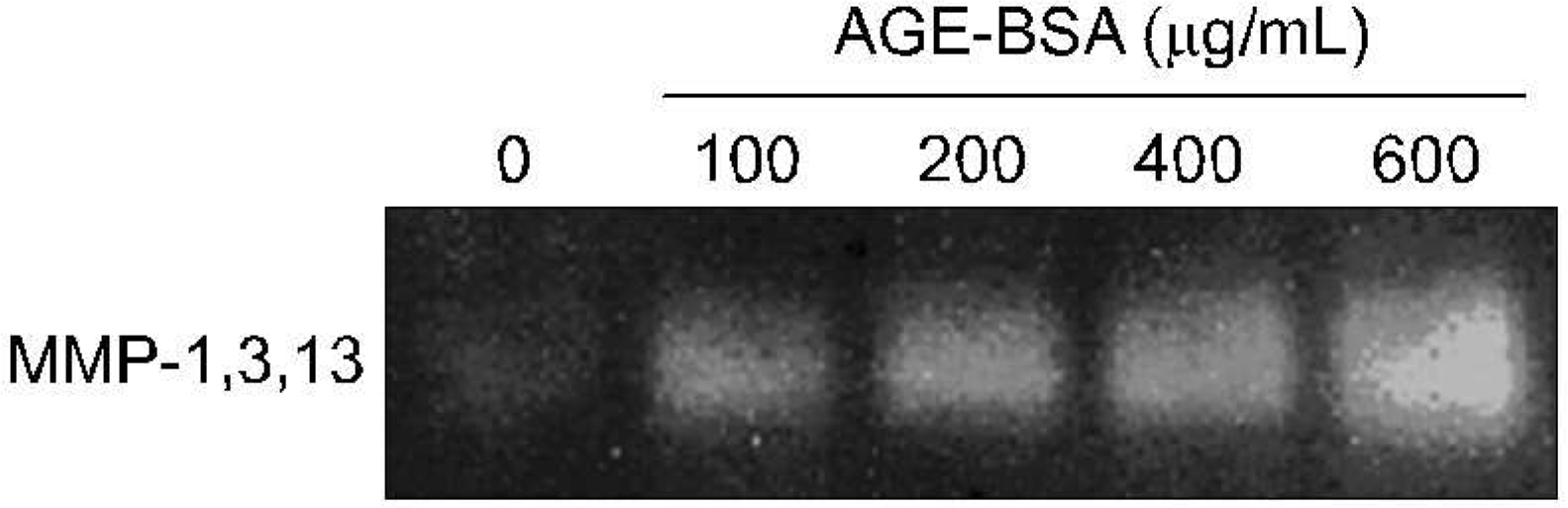 | Fig. 3.Functional assay with zymography on matrix me- talloproteinase (MMPs) activity by advanced gly- cation end products (AGE) in human osteoarthritis (OA) chondrocytes. As MMP-1, 3, and 13 could be expressed in casein gels and have similar size, they were represented like one band. |
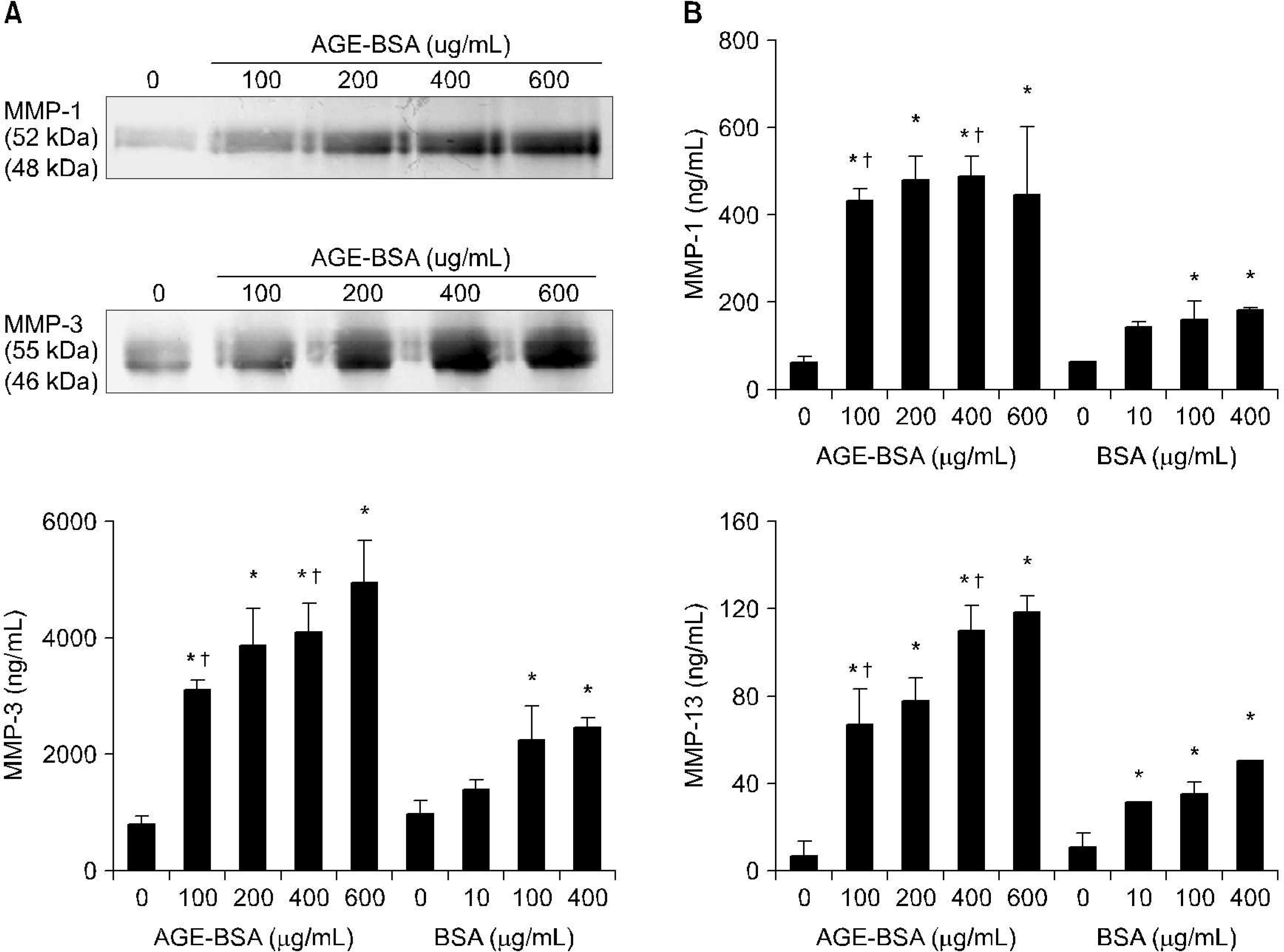 | Fig. 4.Induction of expression of matrix metalloproteinases (MMPs)-1, -3, and-13, by advanced glycation end product bovine serum albumin (AGE-BSA). The expression levels of MMP-1, -3, and-13 were also analyzed by Western-blot assays (A). Enzyme linked immunosorbent assay (ELISA) performed for productions of MMP-1 (upper panel), MMP-3 (middle panel), and MMP-13 (lower panel) (B). Results are representative of duplicate experiments. n=6, ∗p<0.05. compaired with none and AGE-BSA or BSA treatment. p<0.05. compaired with same dose of BSA. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download