REFERENCES
1). Hooyman JR., Melton LJ 3rd., Nelson AM., O'Fallon WM., Riggs BL. Fractures after rheumatoid arthritis. A population-based study. Arthritis Rheum. 1984. 27:1353–61.

3). Spector TD., Hall GM., McCloskey EV., Kanis JA. Risk of vertebral fracture in women with rheumatoid arthritis. Br Med J. 1993. 306:558.

4). Dequeker J., Maenaut K., Verwilghen J., Westhovens R. Osteoporosis in rheumatoid arthritis. Clin Exp Rheumatol. 1995. 13((Suppl 12):):S21–6.
5). Lane NE., Pressman AR., Star VL., Cummings SR., Nevitt MC. Rheumatoid arthritis and bone mineral density in elderly women. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1995. 10:257–63.
6). Cortet B., Flipo RM., Pigny P., Duquesnoy B., Boersma A., Marchandise X, et al. Is bone turnover a determinant of bone mass in rheumatoid arthritis? J Rheumatol. 1998. 25:2339–44.
7). Gough A., Sambrook P., Devlin J. Osteoclastic activation is the principal mechanism leading to secondary osteoporosis in rheumatoid arthritis. J Rheumatol. 1998. 25:1282–9.
8). Laan RF., van Riel PL., van de Putte LB. Bone mass in patients with rheumatoid arthritis. Ann Rheum Dis. 1992. 51:826–32.

9). Gough AK., Lilley J., Eyre S., Holder RL., Emery P. Generalized bone loss in patients with early rheumatoid arthritis. Lancet. 1994. 344:23–7.
10). Kong YY., Feige U., Sarosi I. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999. 402:304–9.

11). Walsh NC., Gravallese EM. Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr Opin Rheumatol. 2004. 16:419–27.

12). Gravallese EM., Harada Y., Wang JT. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998. 152:943–51.
13). Ritchlin CT., Haas-Smith SA., Li P. Mechanisms of TNF-alpha-and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003. 111:821–31.
14). Itonaga I., Fujikawa Y., Sabokbar A., Murray DW., Athanasou NA. Rheumatoid arthritis synovial macro-phage osteoclast differentiation is osteoprotegerin ligand-dependent. J Pathol. 2000. 192:97–104.
15). Hirayama T., Danks L., Sabokbar A., Athanasou NA. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis Rheumatology. 2002. 41:1232–9.
16). Molenaar ET., Voskuyl AE., Dinant HJ., Bezemer PD., Boers M., Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004. 50:36–42.

17). Van Oosterhout M., Levarht EW., Sont JK., Huizinga TW., Toes RE., van Laar JM. Clinical efficacy of infliximab plus methotrexate in DMARD naive and DMARD refractory rheumatoid arthritis is associated with decreased synovial expression of TNF alpha and IL18 but not CXCL12. Ann Rheum Dis. 2005. 64:537–43.
18). Redlich K., Hayer S., Maier A. Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 2002. 46:785–92.
19). Sims NA., Green JR., Glatt M., Schlict S., Martin TJ., Gillespie MT., Romas E. Targeting osteoclasts with zoledronic acid prevents bone destruction in collagen-induced arthritis. Arthritis Rheum. 2004. 50:2338–46.

20). Pacifici R., Carano A., Santoro SA., Rifas L., Jeffrey JJ., Malone JD, et al. Bone matrix constituents stimulate interleukin-1 release from human blood mononuclear cells. J Clin Invest. 1991. 87:221–8.

21). Breuil V., Cosman F., Stein L., Horbert W., Nieves J., Shen V, et al. Human osteoclast formation and activity in vitro: effects of alendronate. J Bone Miner Res. 1998. 13:1721–9.

22). O'Gradaigh D., Ireland D., Bord S., Compston JE. Joint erosion in rheumatoid arthritis: interactions between tumour necrosis factor alpha, interleukin 1, and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclasts. Ann Rheum Dis. 2004. 63:354–9.
23). Pettit AR., Ji H., von Stechow D., Muller R., Goldring SR., Choi Y, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001. 159:1689–99.

24). Herrak P., Gortz B., Hayer S., Redlich K., Reiter E., Gasser J, et al. Zoledronic acid protects against local and systemic bone loss in tumor necrosis factor-mediated arthritis. Arthritis Rheum. 2004. 50:2327–37.

25). Sauty A., Pecherstorfer M., Zimmer-Roth I., Fioroni P., Juillerat L., Markert M, et al. Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonates treatment in vitro and inpatients with malignancy. Bone. 1996. 18:133–9.
26). Thiebaud D., Sauty A., Burckhardt P., Leuenberger P., Sitzler L., Green JR, et al. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int. 1997. 61:386–92.
27). Papadaki HA., Tsatsanis C., Christoforidou A., Malliaraki N., Psyllaki M., Pontikoglou C, et al. Alendronate reduces serum TNFalpha and IL-1beta, increases neutrophil counts, and improves bone mineral density and bone metabolism indices in patients with chronic idiopathic neutropenia (CIN)-associated osteopenia/osteoporosis. J Bone Miner Metab. 2004. 22:577–87.
28). Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000. 408:600–5.
29). Udagawa N. The mechanism of osteoclast differentiation from macrophages: possible roles of T lymphocytes in osteoclastogenesis. J Bone Miner Metab. 2003. 21:337–43.

Fig. 1.
RT-PCR for the osteoclastogenesis related cytokine mRNA from PBMCs of 12 patients with RA with low BMD and 12 age-matched normal individuals· The expression of osteoclastogenesis related cytokine mRNA was not different between the PBMCs of 12 patients with RA with low BMD and 12 age-matched normal individuals· The amount of the cytokine expression varied too much between individuals to compare each other in each group· The PCR condition was initial 10 minutes at 95oC, 35 cycles at 95oC for 30 seconds and 56oC for 60 seconds, and last 10 minutes at 72oC·
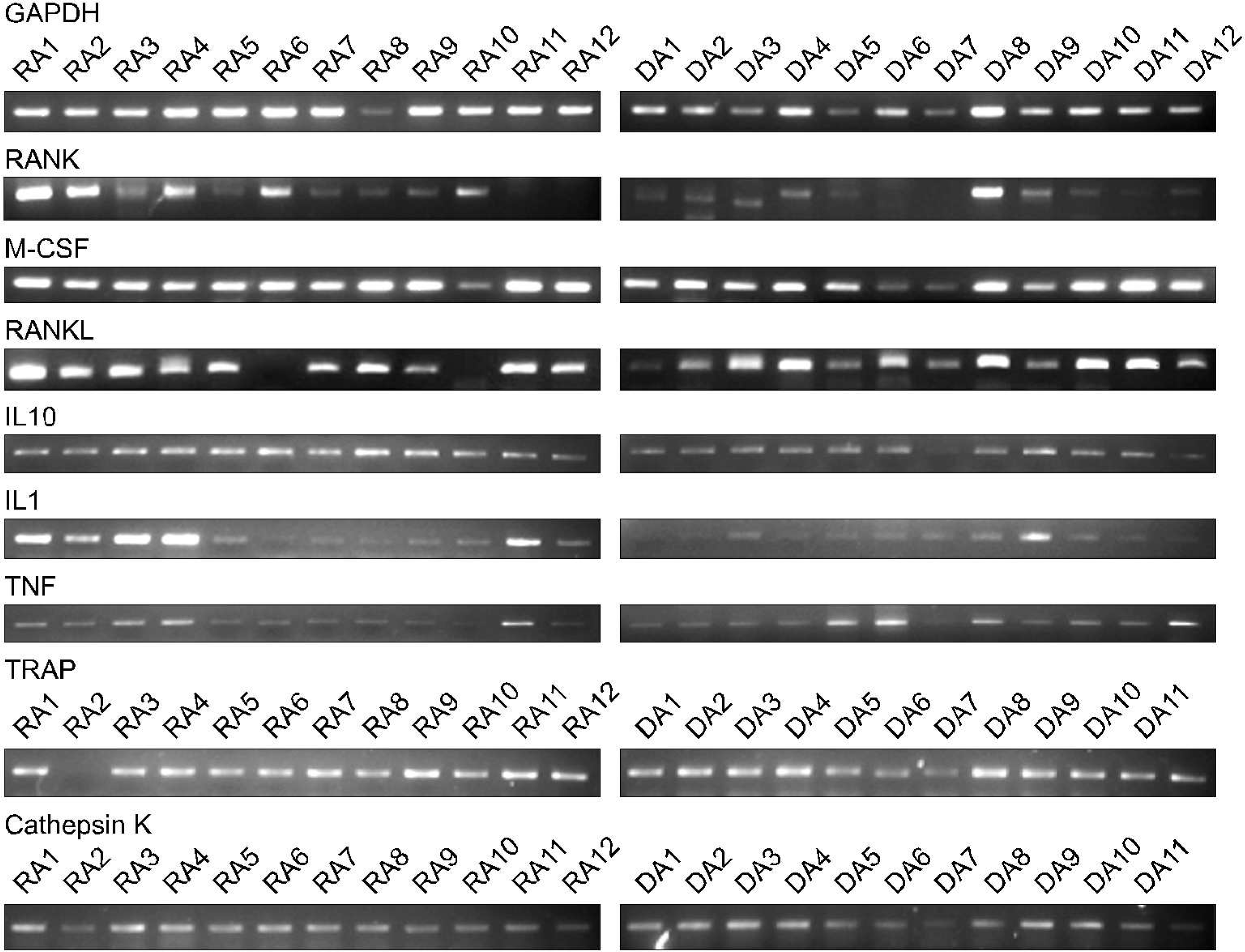
Fig. 2.
After culture the osteoclast from a patient with the RA (X200, light microscope, Counter stain with the Hematoxylin). TRAP positive giant cells with more than 3 nuclei were regarded as osteoclast. TRAP positive giant cells were generated from the human PBMCs after 3 weeks of culture (X400).
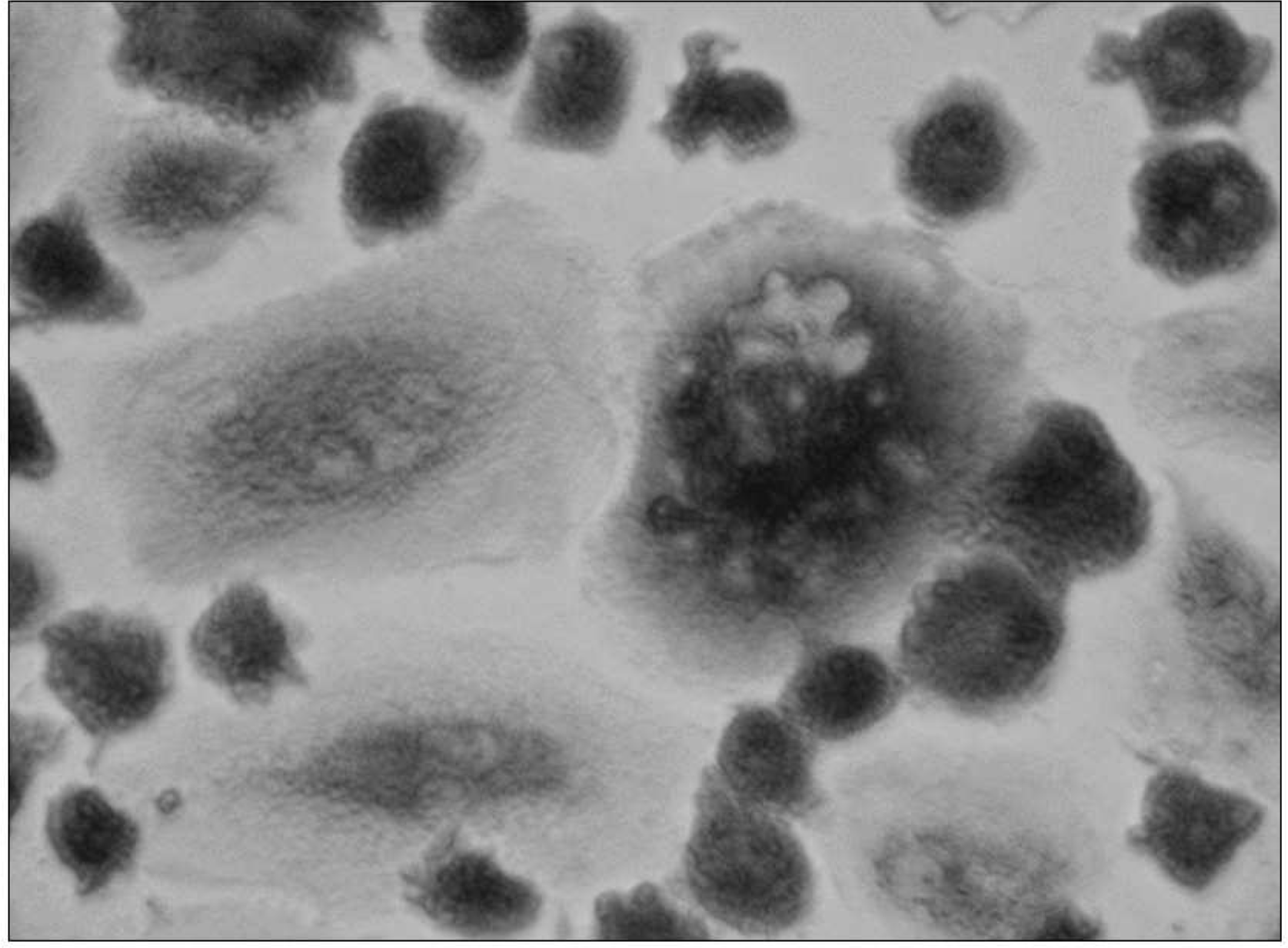
Fig. 3.
The number of the TRAP+ giant cell formation from PBMCs of 12 patients with RA with low BMD and 12 age-matched control individuals. They were not different between the groups (p= 0.117). Cell counts are per each well in 96-well culture plate expressed as mean±SD of triplicate culture.
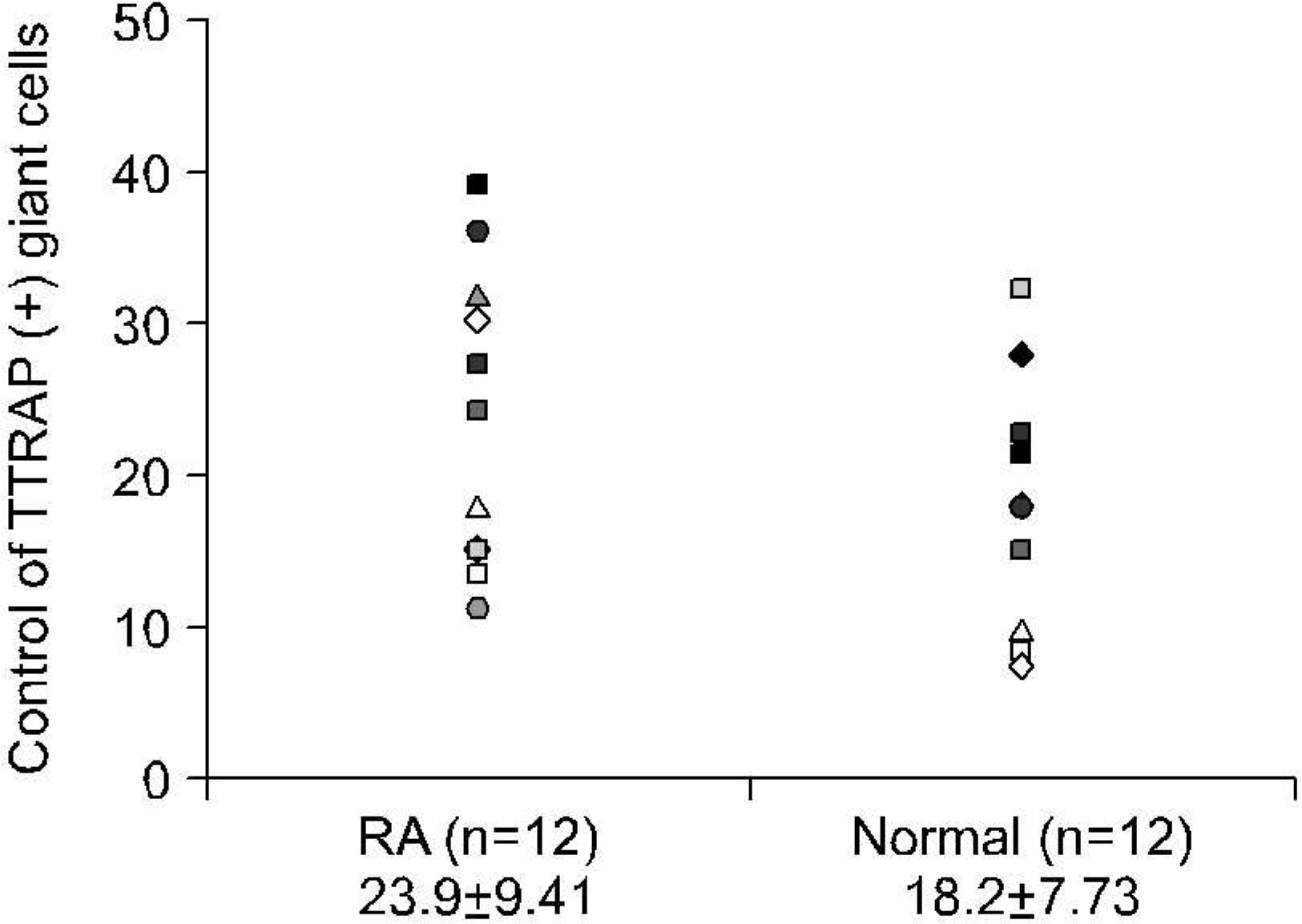
Fig. 4.
Resorption pits were examined under magnifying glass and the percentage surface area of lacunar resorption on each dentine slice was determined as manufacturer's guide. Grade 0: 0%, Grade 1: 20%, Grade 2: 40%, Grade 3: 60%, Grade 4: 80%, Grade 5: 100% (X10).
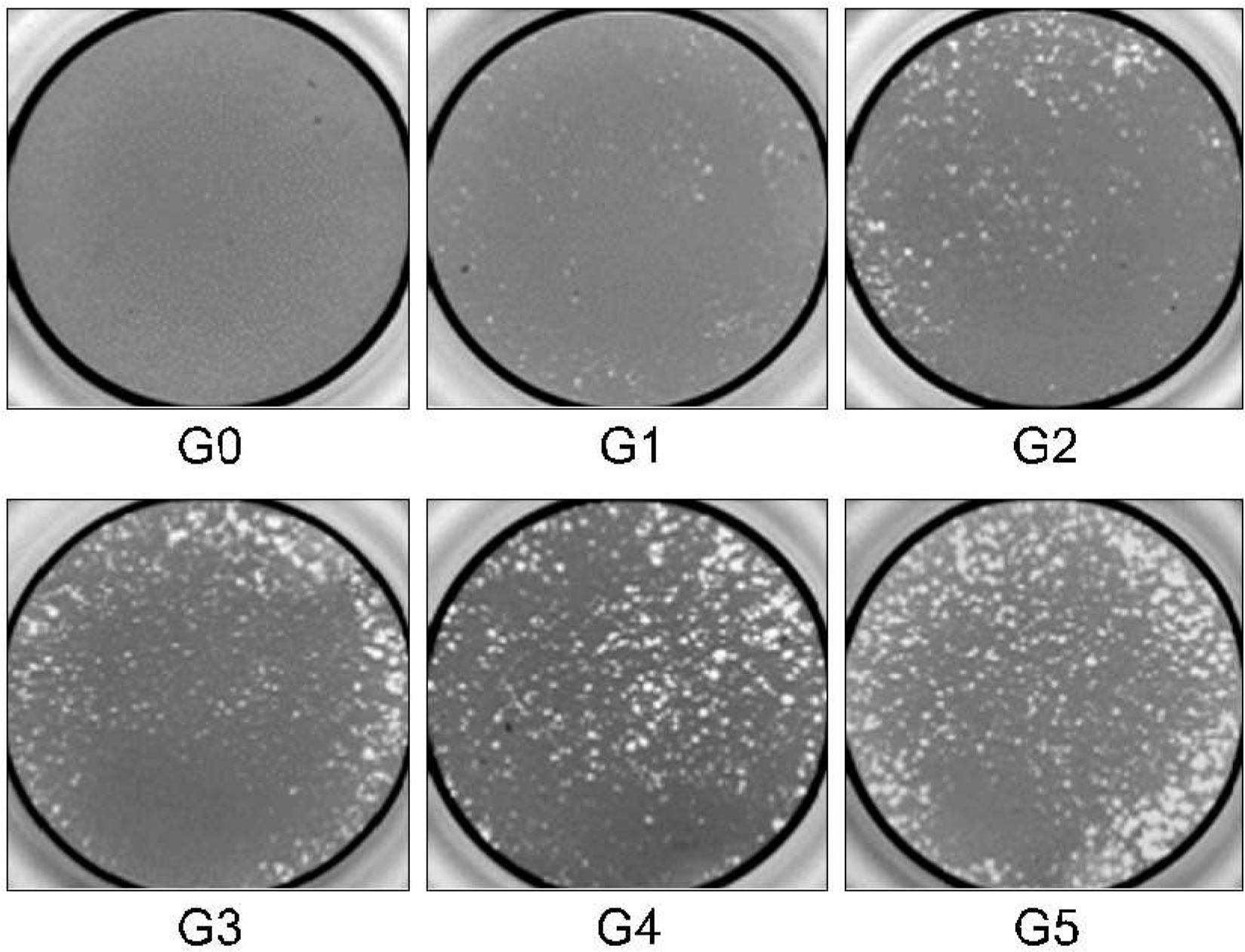
Fig. 5.
The effect of the 18 hours alendronate pulse on the subsequent expression of the osteoclastogenesis related cytokines in a normal control· The increased IFN- γ expression and decreased RANK and IL-10 expression is noted·
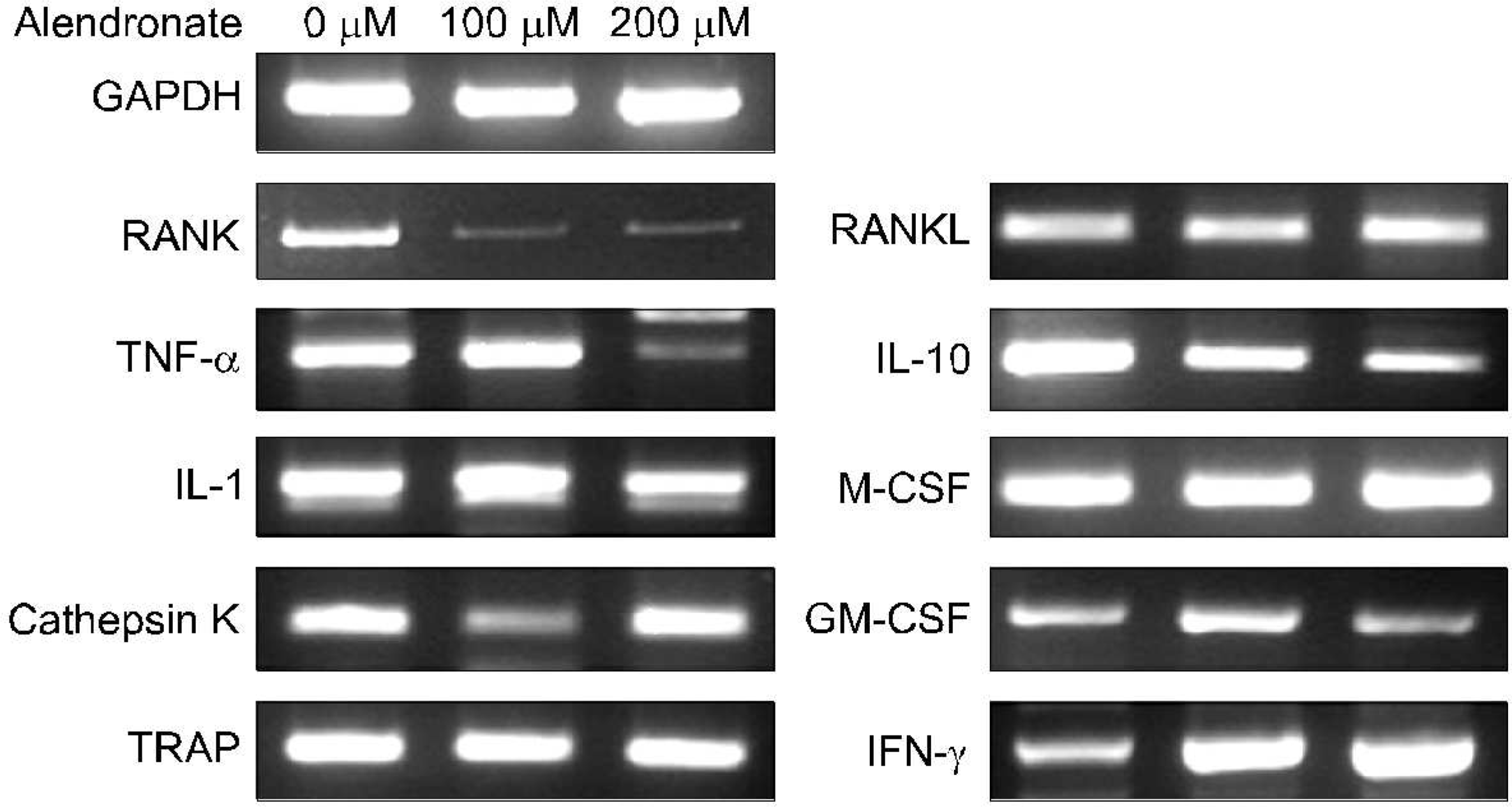
Fig. 6.
(A) The culture of the whole PBMCs for 3 weeks showed many TRAP (+) giant cells (X400). (B) The pre-incubation of the whole PBMCs with the alendronate (100 M) for 24 hours before plating on the culture plate inhibited the development of the TRAP (+) giant cells almost completely (X400).
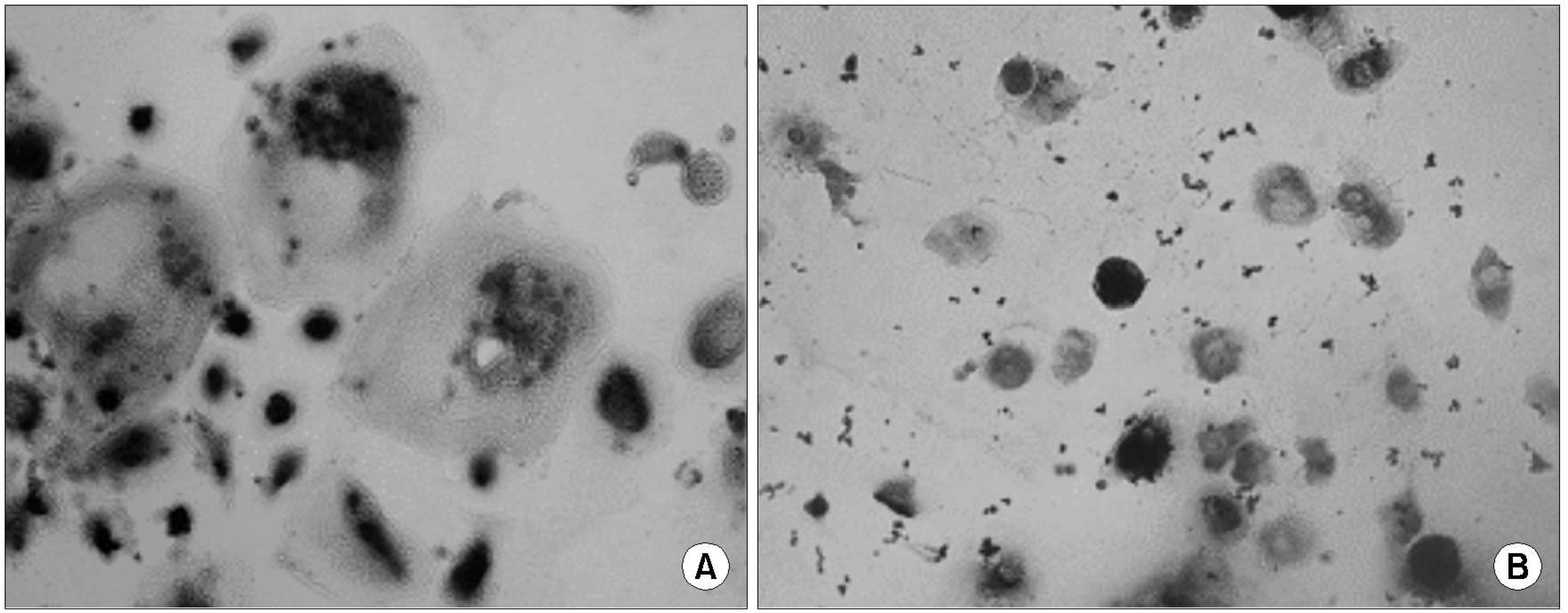
Fig. 7.
(A) The culture of the whole PBMCs on the OAAS™ plate for 4 weeks showed the many resorption pits, Grade 2 (X100). (B) The pre-incubation of the whole PBMCs with the alendronate (100 M) for 24 hours before plating on the OAAS™ plate inhibited the development of the resorption pits almost completely (X100).

Table 1.
Response of osteoclastogenesis related gene expression in PBMCs after 24 hours of alendronate (100 microM) incubation· The real time RT-PCR showed that the mRNA expression of the M-CSF, RANKL, TNF- α, IL-6, and IFN- γ increased· Among them the increase of IFN- γ was most prominent· But those of the RANK, TRAP, and cathepsin K decreased after alendronate pulse of the PBMCs· The IL-1 expression did not changed significantly· The Results of Real Time RT-PCR by “2-zMCT Method”
Table 2.
The count of the TRAP positive cells in 96 well culture plates before and after alendronate pulse· They were not different between the groups (p= 0·117)· The decrease of the TRAP (+) giant cells after alendronate pulse was almost com- plete· The developments of the TRAP (+) giant cells were inhibited markedly after alendronate pulse, but less markedly in RA
| Alendronate dose | RA (n=6) | Age matched control (n=6) | Young normal (n=6) |
|---|---|---|---|
| 0 μM | 23.9±9.41 | 22.8±6.31 | 18.2±7.73 |
| 100 μM | 7.1±4.12 | 0.5±0.84 | 0 |
| 200 μM | 1.8±0.75 | 0 | 0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download