Abstract
Objective:
To determine phenotypic and functional characteristics of memory B cells in patients with systemic lupus erythematosus (SLE).
Methods:
The percentage of memory B cell subsets in peripheral blood mononuclear cells (PBMC) from normal control (n=11), inactive (n=15) and active (n=10) SLE patients was determined by Fluorescence Activated Cell Sorter (FACS). In addition, the activation status of memory B cells was measured by the surface expression of CD86 (B7-2). The production of antibodies to chromatin and dsDNA (IgG and IgM type) by isolated memory B cell subsets was examined by enzyme-linked immunosorbent assay (ELISA).
Results:
In this study, we analyzed 2 subtypes of memory B cells: FSC (Forward Side Scat- ter)low and FSChigh memory B cell. The percentage of both subtypes from active and inactive SLE patients was significantly reduced compared to that of normal controls (p<0.01). In addition, the expression of activation markers, CD86 on FSChigh memory B cells from active SLE patients was higher than those of inactive SLE patients and normal controls (p=0.014). Upon stimulation with CpG and IL-15 in vitro for 8 days, isolated FSChigh memory B cells from active SLE patients revealed augmented production of autoantibodies to chromatin and dsDNA.
REFERENCES
1). Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nature Immunol. 2001. 2:764–6.

2). Thomas HW., Holger F., Joachim R., Kalden . Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol. 1992. 22:1718–28.
3). Gregory CI., Robert LS., Michael Z., Ivaylo I., Ryoki K., Cosima Z, et al. Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production. J Exp Med. 2006. 203:1567–78.
4). Wellman U., Letz M., Hermann M., Angermuller S., Kalden JR., Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci USA. 2005. 102:9258–63.
5). Mark JS., Ann HA., David SP., Martin GW. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. PNAS. 1987. 84:9150–4.
6). Dan E., Malka H., Francois T., Laurent J., Jean-Francois B. The VH gene sequences of anti-DNA antibodies in two different strains of lupus prone mice are highly related. Eur J Immunol. 1989. 19:1241–6.
7). Marion TN., Tillman DM., Jou NT. Interclinal and intraclonal diversity among anti-DNA antibodies from (NZBxNZW) F1 mouse. J Immunol. 1990. 145:2322–32.
8). Louise J., Michael G. Antigen-Specific Memory B cell development. Annu Rev Immunol. 2005. 23:487–513.
9). Neal NI., Ann HL., Prasanth V., Kevin LO., Klaus R., Laurie HG. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nature immunology. 2003. 4:321–9.
10). Bernasconi NL., Traggiai E., Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002. 298:291–4.

11). Wirths S., Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol. 2005. 35:3433–41.
12). Jacobi AM., Odendahl M., Reiter K., Bruns A., Burmester GR., Radbrunch A, et al. Correlation between circulating CD27high plasma cells and disease activity in patients activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003. 48:1332–42.
13). Sato S., Fujimoto M., Hasegawa M., Takehara K. Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum. 2004. 50:1918–27.

Fig. 1.
Frequency of B cell subsets in human PBMC CD19+, CD27- naïve B cells, CD19+, CD27+ memory B cell, and CD19, CD27++ plasma cells were determined in patients with active and inactive SLE and in healthy controls by flow cytometric analysis using 2 different gates; Lymphocyte gate (FSClow) and monocyte gate (FSChigh).
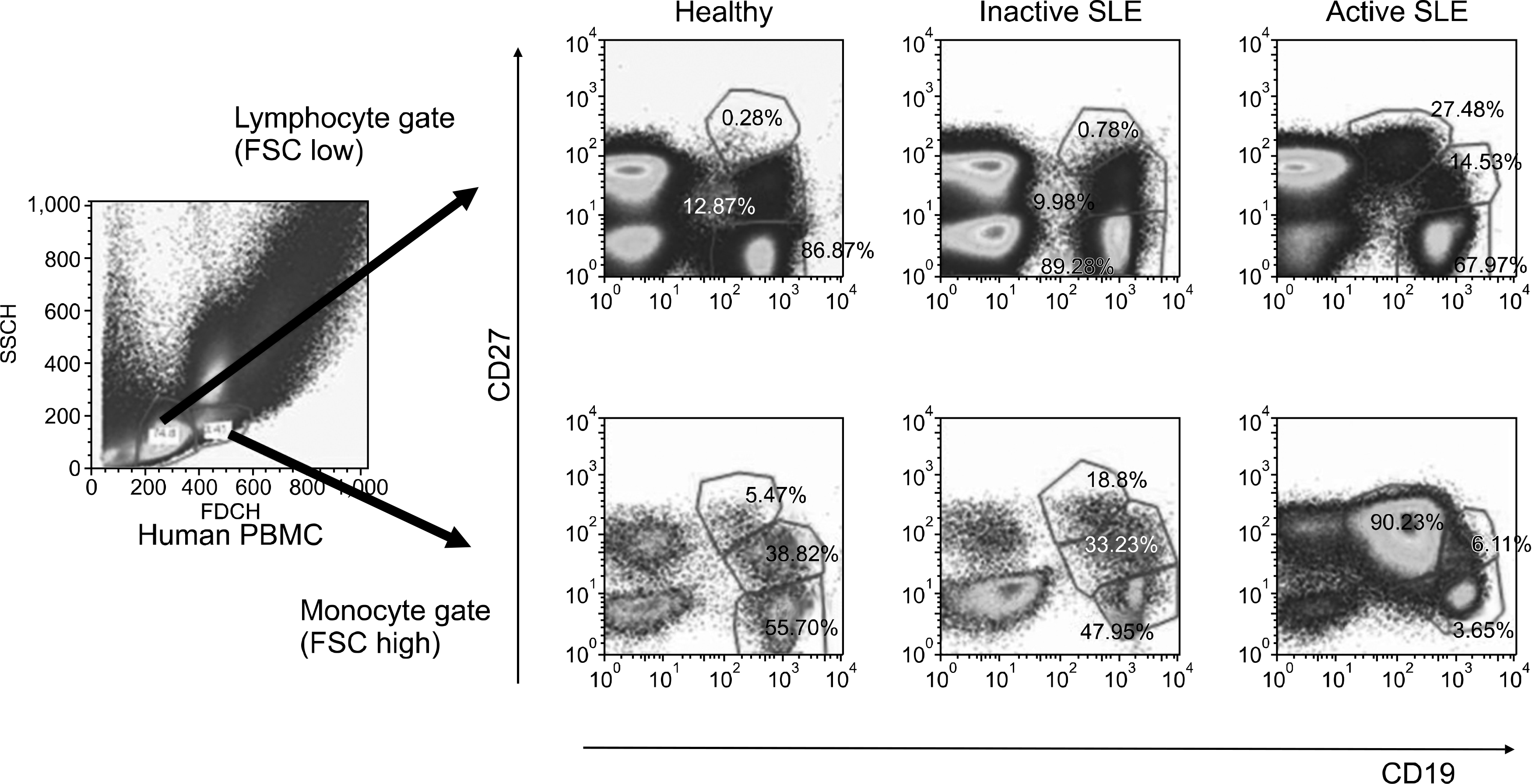
Fig. 2A.
CD86 expression on the memory, naïve and plasma cells from normal control and inactive lupus patients. one of three independent experiments is shown.
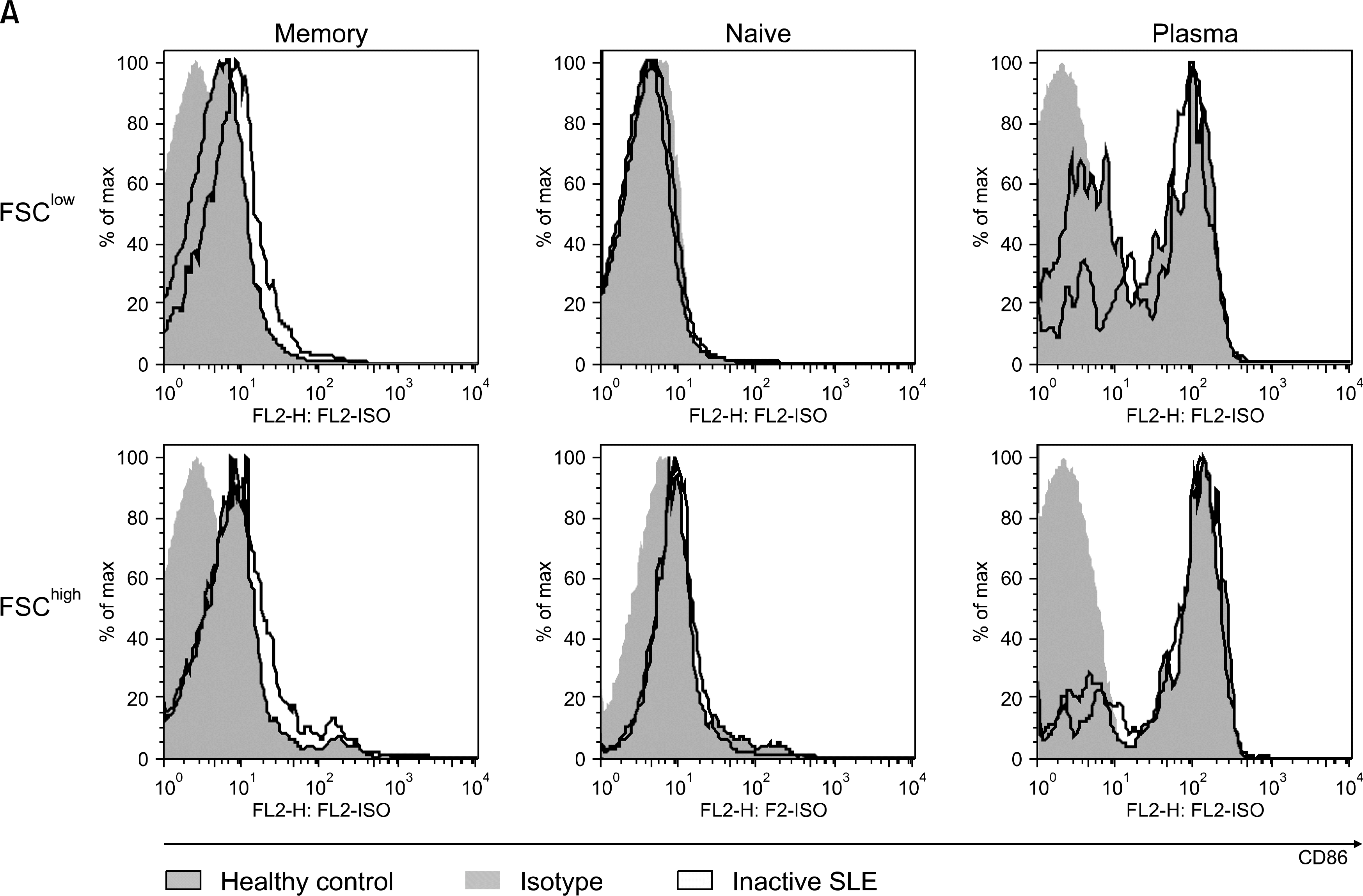
Fig. 2B.
CD86 expression on the memory, naïve and plasma cells from normal control and active lupus patients. one of three independent experiments is shown.
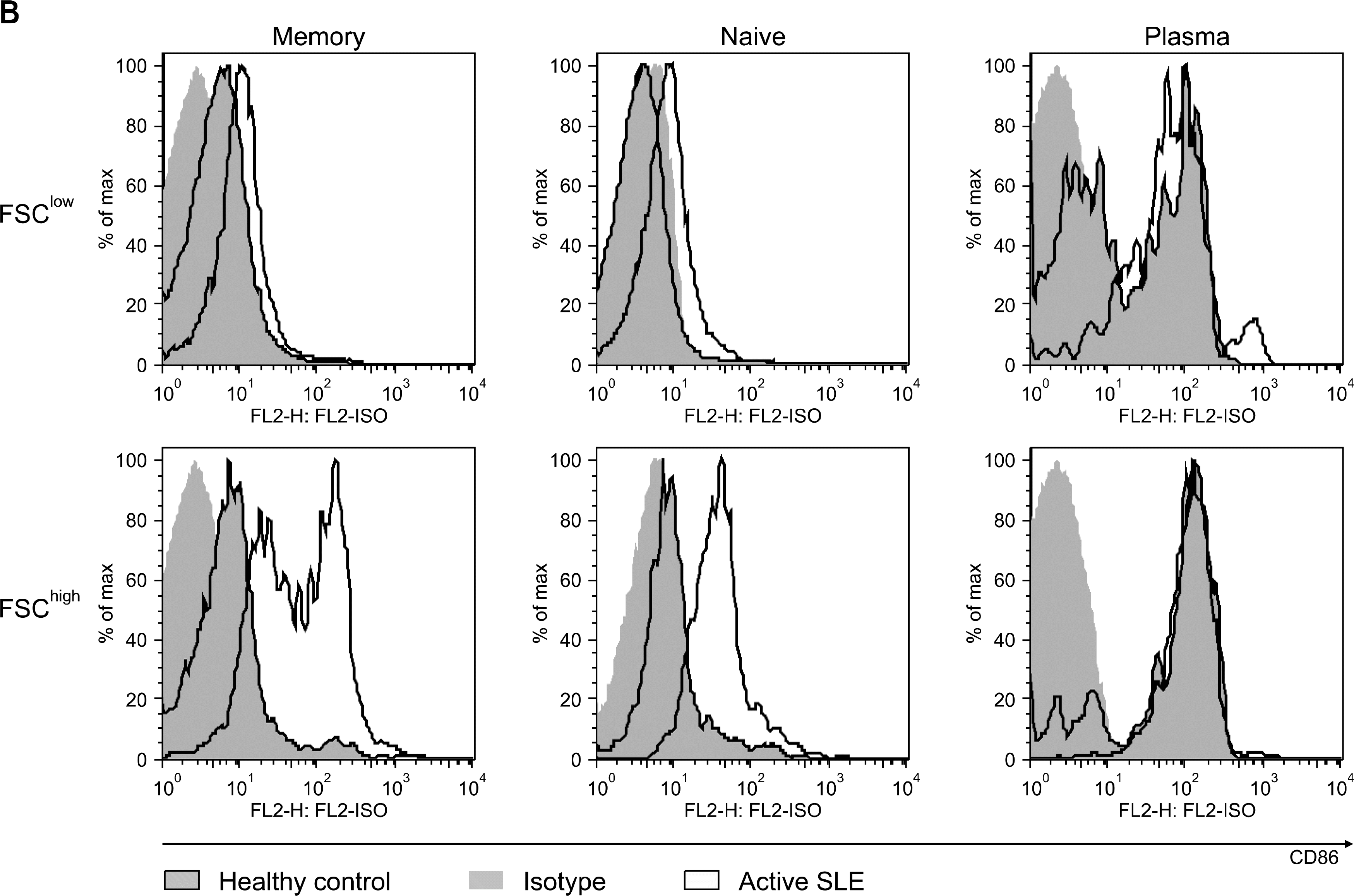
Fig. 3.
Autoantibody production by FSC g memory B cells stimulated with CpG and IL-15 for 8 days in vitro each histogram shows the mean results obtained from each group. ∗(normal healthy supernatant).
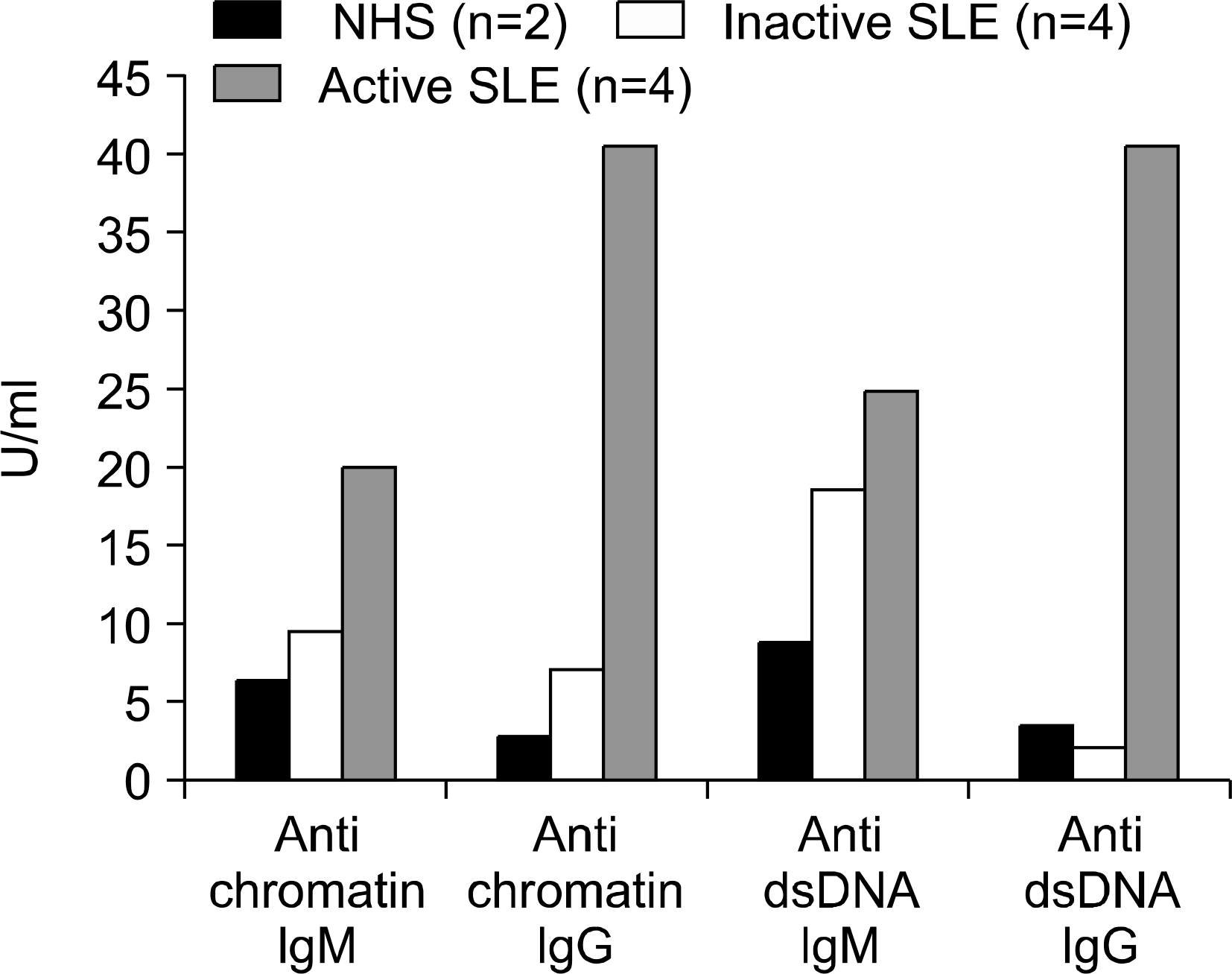
Table 1.
Frequency of plasma cells from healthy, inactive SLE and active SLE
∗ Significant difference between healthy and inactive SLE(p<0.01), ∗∗No Significant difference between healthy and active SLE (p=0.065), ∗∗∗Significant difference between healthy and active SLE (p<0.001), +Nosignifi- cant difference between inactive and active SLE (p= 0.119), Significant difference between inactive and active SLE (p< 0.001)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download