Abstract
Objective:
Hemophilic arthropathy, which results from recurrent intra-articular bleeding, is a proliferative synovitis, but the sequence of pathogenic events in hemophilic synovitis (HS) is not known in detail. To investigate the pathogenic mechanism of HS and to evaluate the suppressive effect of rifampicin for HS, we designed this study.
Methods:
Twenty normal male New Zealand white rabbits weighing approximately 2,000 gm were used for this study. We injected 1 mL of autologous whole blood of the rabbits into the right knee joint and normal saline into the left knee joint (control) thrice a week for 10 weeks and sacrificed 10 of them. We injected 10 mg of rifampicin into the right knee joint of HS, which is 5 rabbits of remained 10, once a week from 11th week until 15th week. At 11th week and 20th week, the rabbits were sacrificed and both knee joints of each rabbit were opened, synovial membrane specimens were collected and examined pathologically and biologically such as mRNA of IL-1, TNF-α, MMP-1 and MMP-3 in hemophilic synovium using by real-time quantitative PCR method (comparative Ct method).
Results:
At 20th week, in rifampicin treated group showed decreased proliferative, and infiltrated mononuclear cells compared with control group. And mRNA of TNF-α, IL-1 and MMP-3 levels were decreased also.
Conclusion:
In animal model of HS, histological changes showed the same as human hemophilic synovitis. And this study suggested that rifampicin has a controlling effect on the inflammatory process of HS by suppression of inflammatory cytokine production in the experimental hemophilic synovitis model.
Go to : 
REFERENCES
1). Rodriguez-Merchan EC. Pathogenesis, early diagnosis, and prophylaxis for chronic hemophilic synovitis. Clin Orthop. 1997. 343:6–11.
2). Arnold WD., Hilgartner MW. Haemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977. 59:287–305.
4). Aledort LM., Haschmeyer RH., Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Int Med. 1992. 236:391–9.
5). Petrini P., Lindvall N., Egberg N., Blomback M. Prophylaxis with factor concentrates in preventing haemophilic arthopaghy. Am J Pediatr Hematol Oncol. 1991. 13:280–7.
6). Soreff J., Blomback M. Arthropathy in children with severe hemophilia A. Acta Paedriatr Scand. 1980. 69:667–73.

7). Roosendaal G., Vianen ME., Wenting MJ., van Rinsum AC., van den Berg HM., Lafeber FP, et al. Iron deposits and catabolic properties of synovial tissue from patients with haemophilia. J Bone Joint Surg Br. 1998. 80:540–5.

8). Rodriguez-Merchan EC. Methods to treat chronic haemophilic synovitis. Ann Rheum Dis. 1991. 50:588–91.

9). Roosendaal G., Mauser-Bunschoten EP., de Kleijn P., Heijnen L., van den Berg HM., Van Rinsum AC, et al. Synovium in haemophilic arthropathy. Haemophilia. 1998. 4:502–5.
10). Hooiveld MJ., Roosendaal G., Jacobs KM., Vianen ME., van den Berg HM., Bijlsma JW, et al. Initiation of degenerative joint damage by experimental bleeding combined with loading of the joint: a possible mechanism of hemophilic arthropathy. Arthritis Rheum. 2004. 50:2024–31.

11). Roosendaal G., Vianen ME., van den Berg HM., Lafeber FP., Bijlsma JW. Cartilage damage as a result of hemarthrosis in a human in vitro model. J Rheumatol. 1997. 24:1350–4.
12). Roosendaal G., Vianen ME., Marx JJ., van den Berg HM., Lafeber FP., Bijlsma JW. Blood-induced joint damage: a human in vitro study. Arthritis Rheum. 1999. 42:1025–32.

13). Caviglia HA., Fernandez-Palazzi F., Galatro G., Perez-Bianco R. Chemical synoviorthesis with rifampicin in hemophilia. Hemophilia. 2001. 7:26–30.
14). Caviglia HA., Fernandez-Palazzi F., Maffei E., Galatro G., Barrionuevo A. Chemical synoviorthesis for hemophilic synovitis. Clin Orthop Relat Res. 1997. 343:30–6.

15). Stein H., Duthie RB. The pathogenesis of chronic haemophilic arthropathy. J Bone Joint Surg Br. 1981. 63-B:601–9.

16). Erken EHW. Radiocolloids in the management of hemophilic arthropathy in children and adolescents. Clin Orthop. 1991. 264:129–35.

17). Fernandez-Palazzi F., de Bosch NB., de Vargas AF. Radioactive synovectomy in haemophilic haemarthrosis. Follow-up of fifty cases. Scand J Haematol. 1984. 40(Suppl):s291–s300.

18). Fernandez-Palazzi F., Rivas S., Cibeira JL., Dib O., Viso R. Radioactive synoviorthesis in hemophilic synovitis. Materials, techniques, and dangers. Clin Orthop. 1996. 328:14–8.
19). Greene WB: Synovectomy of the ankle for hemophilic arthropathy. J Bone Joint Surg. 1994. 76A:812–9.
20). Mannucci PM., DeFranchis R., Torri G., Pietrogrande V. Role of synovectomy in hemophilic arthropathy. Isr J Med Sci. 1977. 13:983–7.
21). Matsuda Y., Duthie RB. Surgical synovectomy for haemophilic arthropathy of the knee. Long-term follow-up. Scand J Haematol. 1984. 40((Suppl):):237–47.
Go to : 
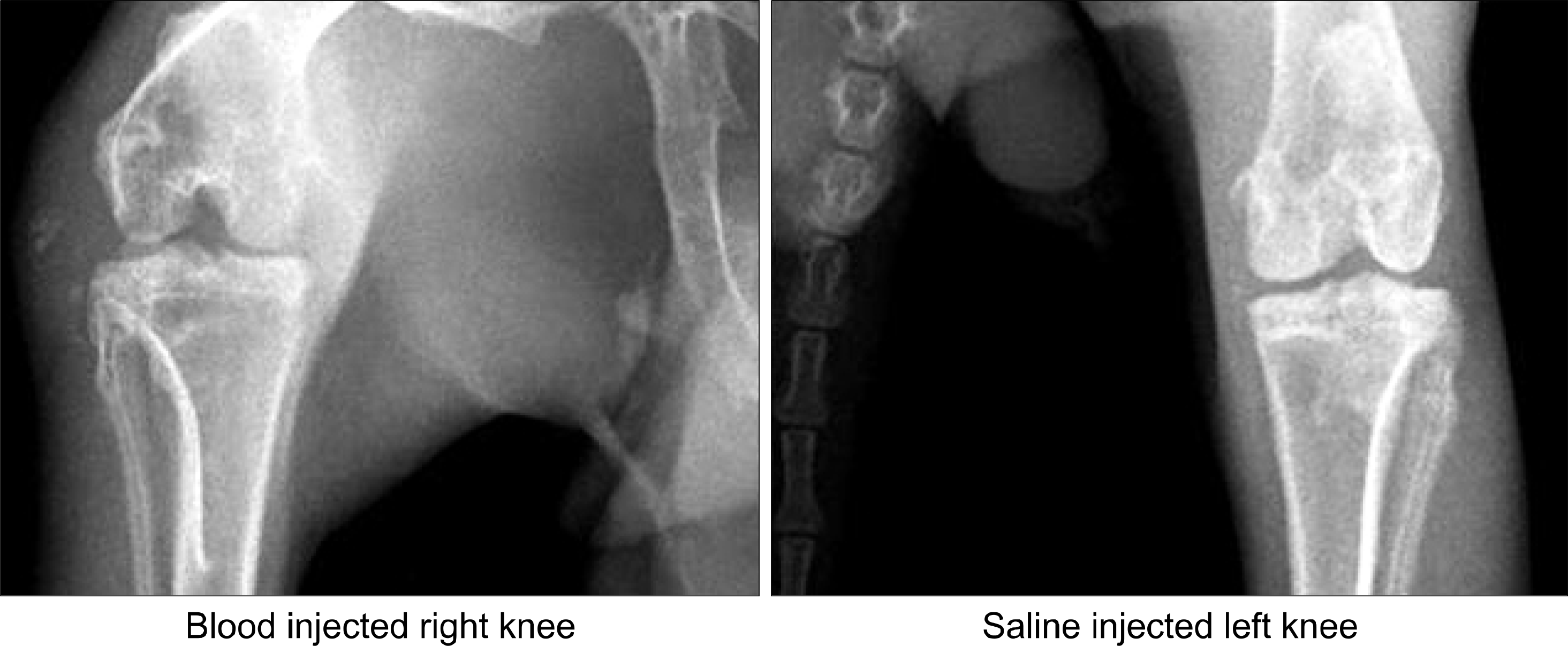
 | Fig. 1.The blood injected knee of rabbit (arrow) was more swollen compared with the other knee. |
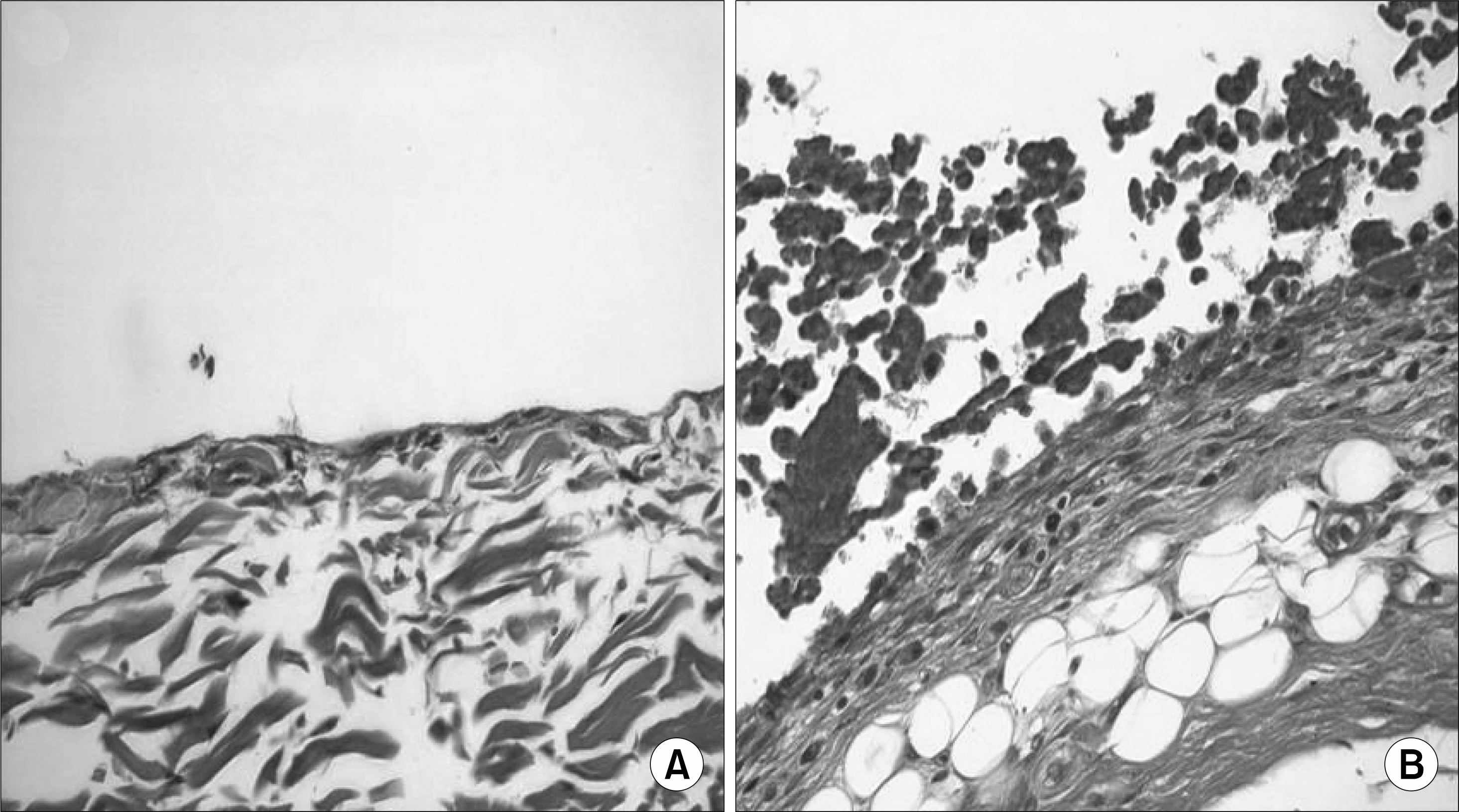 | Fig. 3.The synovium of blood injected knee shows proliferation and fat tissue was increased under synovium (A) normal saline injected knee synovium (B) blood injected knee synovium (H&E stain, x 400). |
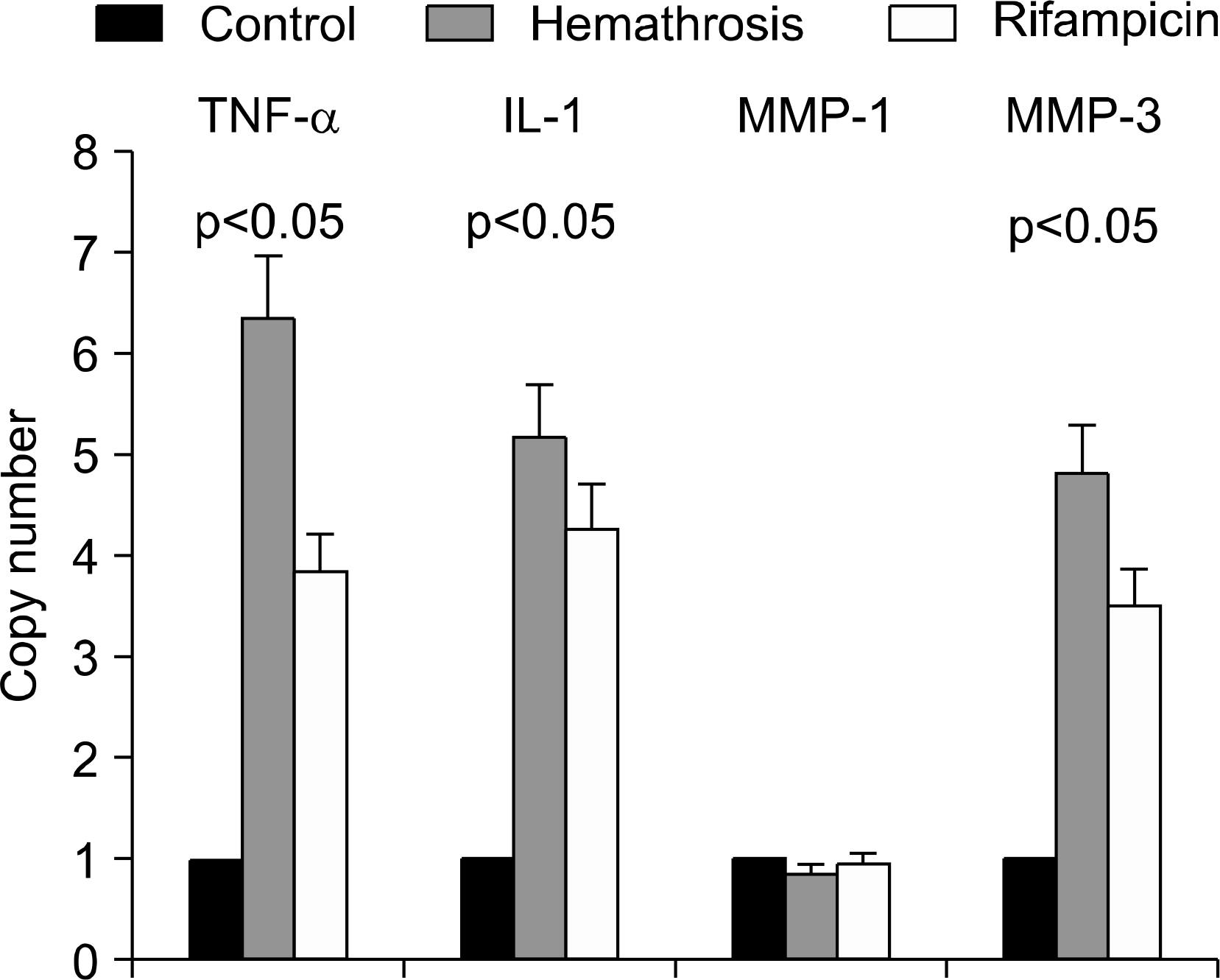 | Fig. 4.The expression of TNF-α, IL-1, MMP-1 and MMP-3 mRNA in synovium of animal model of Hemathrosis (HA) by real time-PCR. Control is saline-injected knee, HA is blood-injected synovium without rifampicin, and RFP is blood-injected synovium treated with rifampicin. |
Table 1.
Primer and PCR protocol according to each cytokine




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download