Abstract
Advanced knowledges of cellular and molecular biology led to the development of therapies of rheumatoid arthritis (RA). The introduction of biologic agents into clinical practice has had a profound effect on the current management of RA. These agents can rapidly enhance functional status and inhibit the progression of joint damage from the disease.
Tumor necrosis factor (TNF) inhibitors are the representative biologic agents for the treatment of RA. Their clinical efficacy and safety were revealed through many clinical trails. Novel TNF inhibitors and other biologic agents for the treatment of RA are developing continuously. Promising biologic agents such as TNF inhibitors have become an important part of treatment armamentarium for RA, although they have some side effects and problems to be solved. These agents may contribute to achieve the sustained remission and eventually cure of RA. Currently available TNF inhibitors in Korea include etanercept, infliximab and adalimumab.
This review article introduces past, present, and future of the TNF inhibitors and suggests guidelines for the proper use of them on RA.
REFERENCES
1). Cush JJ., Kavanaugh AF. Biologic interventions in rheumatoid arthritis. Rheum Dis Clin North Am. 1995. 21:797–816.

2). Fox D. Cytokine blockade as a new strategy to treat rheumatoid arthritis. Arch Intern Med. 2000. 160:437–44.

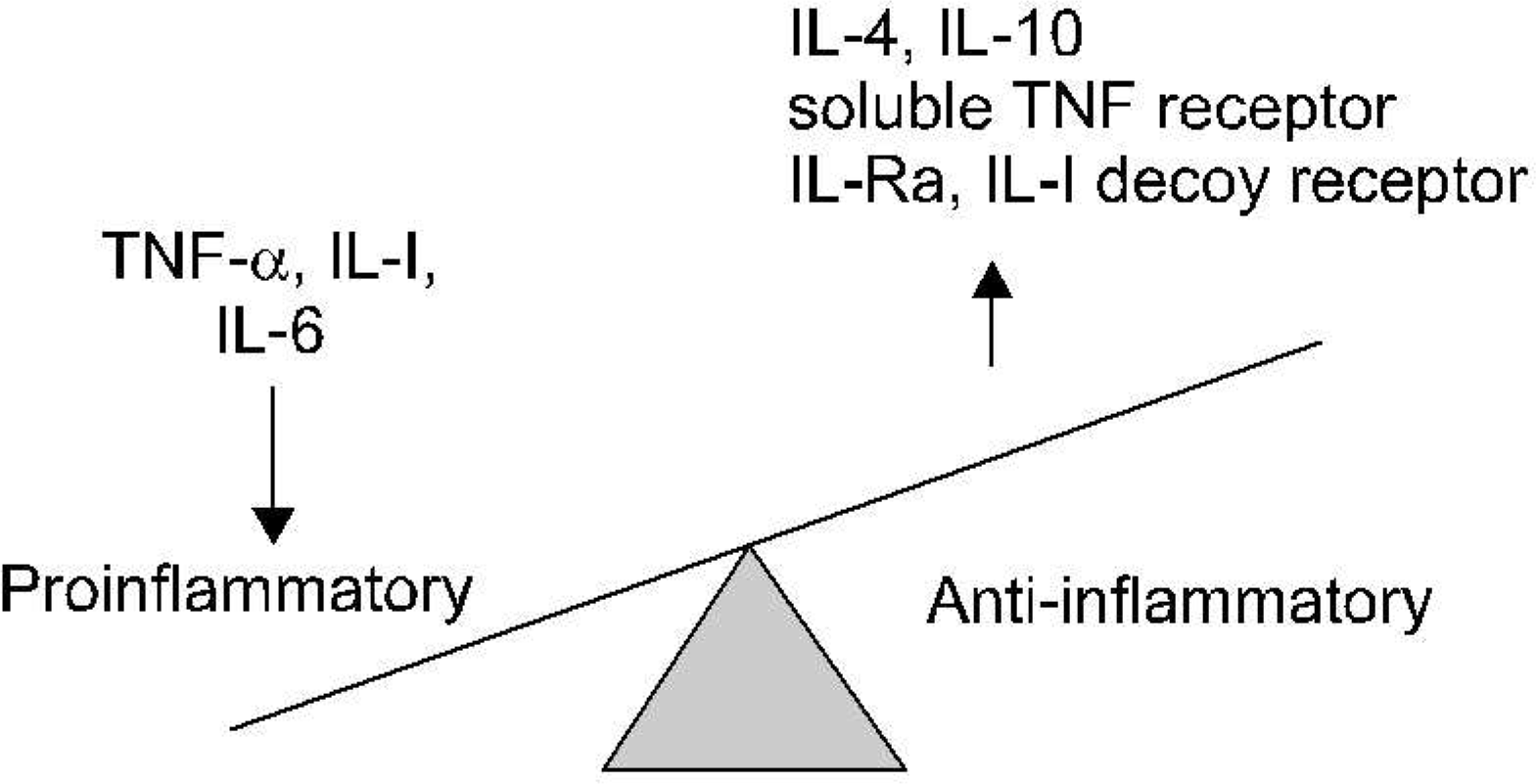
3). Dinarello CA., Moldawer LL. Proinflammatory and anti-inflammatory cytokines in rheumatoid arthritis: a primer for clinicians. 2nd ed.Thousand Oak (CA), Amgen;2000.
4). Chikanza IC., Kingsley G., Panayi GS. Peripheral blood and synovial fluid monocyte expression of interleukin 1 α and 1 α during active rheumatoid arthritis. J Rheumatol. 1995. 22:600–6.
5). Saxne T., Palladino MA Jr., Heinegard D., Talal N., Wollheim FA. Detection of tumor necrosis factor α but not tumor necrosis factor α in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988. 31:1041–5.

6). Shingu M., Nagai Y., Isayama T., Naono T., Nobunaga M., Nagai Y. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol. 1993. 94:145–9.

7). Breedveld FC., Kalden JR. Appropriate and effective management of rheumatoid arthritis. Ann Rheum Dis. 2004. 63:627–33.

8). Bazzoni F., Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996. 334:1717–25.

9). Zhang M., Tracey KJ. Tumor necrosis factor. In:. Thompson A, editor. ed.the cytokine handbook. 3rd ed.p. 517–48. San Diego, Calif: Academic Press;1998.
10). Black RA., Rauch CT., Kozlosky CJ., Peschon JJ., Slack JL., Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997. 385:729–33.

11). Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986. 322:547–9.
12). Bang LM., Keating GM. Adalimumab: a review of its use in rheumatoid arthritis. Bio Drugs. 2004. 18:121–39.
13). Breedveld FC., Emery P., Keystone E., Patel K., Furst DE., Kalden JR, et al. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004. 63:149–55.

14). Klareskog L., van der Heijde D., de Janger JP., Gough A., Kalden J., Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004. 363:675–81.

15). Smolen JS., Han C., Bala M., Maini R., Kalden J., van der Heijde D, et al. Evidence of radiographic benefit of infliximab plus methotrexate in rheumatoid arthritis patients who hand no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum. 2005. 52:1020–30.
16). Breedveld FC., Weisman MH., Kavanaugh AF., Cohen SB., Pavelka K., van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive, rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006. 54:26–37.

17). Genovese MC., Bathon JM., Martin RW., Fleischmann RM., Tesser JR., Schiff MH, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002. 46:1443–50.

18). Keystone EC., Kavanaugh AF., Sharp JT., Tannenbaum H., Hua Y., Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy - a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004. 50:1400–11.
19). Enbrel® [package insert]. Seattle, WA: Immunex Corporation;. 2002.
20). Moreland LW., Schiff MH., Baumgartner SW., Tindall EA., Fleischmann RM., Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis: a randomized controlled trial. Ann Intern Med. 1999. 130:478–86.
21). Moreland LW., ᄋ: Dell JR. Glucocorticoids and rheumatoid arthritis: back to the future? Arthritis Rheum. 2002. 46:2553–63.

22). Klareskog L., van der Heijde D., de Jager JP., Gough A., Kalden J., Malaise M, et al. TEMPᄋ(Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators. Therapeutic effect of the combination of etanercept and metho- trexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004. 363:675–81.
23). 요양급여의적용기준및방법에관한세부사항(약제) 개정고시(보건복지부고시제2006-33호, 06.5.1 시행). http://www.hira.or.kr/cms/rf/rfe/stdinfo_01/1185585_1238.html. 건강보험심사평가원: http://www.hira.or.kr/cms/rf/rfe/stdinfo_01/1185585_1238.html. 건강보험심사평가원:. 2006.
24). Lovell DJ., Giannini EH., Reiff A., Cawkwell GD., Silverman ED., Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000. 342:763–9.
25). Lovell DJ., Giannini EH., Reiff A., Jones OY., Schneider R., Olson JC, et al. Pediatric Rheumatology Collaborative Study Group. Arthritis Rheum. 2003. 48:218–26.
26). Knight DM., Trinh H., Le J., Siegel S., Shealy D., Mc-Donough M, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993. 30:1443–53.

27). Maini RN., Breedveld FC., Kalden JR., Smolen JS., Davis D., Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998. 41:1552–63.
28). Smolen JS., Van Der Heijde DM., St Clair EW., Emery P., Bathon JM., Keystone E, et al. Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset (ASPIRE) Study Group. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006. 54:702–10.
29). 유대현: 박성환: 송관규: 박원: 조철수: 송영욱. 한국인활동성류마티스관절염환자에서인플릭시맵의유효성과안정성. 대한류마티스학회지. 2006. 13(Suppl 2):S39–S47.
30). Gonzalez-Juanatey C., Testa A., Garcia-Castelo A., Garcia-Porrua C., Llorca J., Gonzalez-Gay MA. Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti-tumor necrosis factor - α antibody. Arthritis Rheum. 2004. 51:447–50.
31). Gonzalez-Gay MA., Garcia-Unzueta MT., De Matias JM., Gonzalez-Juanatey C., Garcia-Porrua C., Sanchez-Andrade A, et al. Influence of anti-TNF-alpha infliximab therapy on adhesion molecules associated with atherogenesis in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006. 24:373–9.
32). Remicade® [package insert]. Malvern, PA: Centocor, Inc;. 2006.
33). Schmeling H., Horneff G. Infliximab in two patients with juvenile ankylosing spondylitis. Rheumatol Int. 2004. 24:173–6.

34). Tse SM., Burgos-Vargas R., Laxer RM. Anti-tumor necrosis factor alpha blockade in the treatment of juvenile spondylarthropathy. Arthritis Rheum. 2005. 52:2103–8.
35). Lahdenne P., Vahasalo P., Honkanen V. Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis. 2003. 62:245–7.

36). Tynjala P., Lahdenne P., Vahasalo P., Kautiainen H., Honkanen V. Impact of anti-TNF treatment on growth in severe juvenile idiopathic arthritis. Ann Rheum Dis. 2006. 65:1044–9.

37). Katsicas MM., Russo RA. Use of infliximab in patients with systemic juvenile idiopathic arthritis refractory to etanercept. Clin Exp Rheumatol. 2005. 23:545–8.
38). Humira® [package insert]. North Chicago, IL: Abbott Laboratories. 2003.
39). Salfeld J., Kaymakcalan Z., Tracey D., Robert A., Kamen R. Generation of fully human anti-TNF antibody D2E7 [abstract]. Arthritis Rheum. 1998. 41(Suppl):S57.
40). Rau R. Adalimumab (a fully human anti-tumor necrosis factor α monoclonal antibody) in the treatment of active rheumatoid arthritis: the initial results of five trials. Ann Rheum Dis. 2002. 61(Suppl III):ii70–3.
41). van de Putte LBA., Salfeld J., Kaymakcalan Z. Adalimumab. In: Morelang LW, Emery P, eds. TNF-inhibition in the treatment of rheumatoid arthritis. Martin Dunitz, London. 2003. 71–93.
42). Weinblatt ME., Keystone EC., Furst DE., Moreland LW., Weisman MH., Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003. 48:35–45.
43). Breedveld FC., Weisman MH., Kavanaugh AF., Cohen SB., Pavelka K., van Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006. 54:26–37.

44). Kay J., Matteson EL., Dasgupta B., Nash P., Durez P., Hall S, et al. One-year results of golimumab compared with placebo in patients with active RA despite treatment with methotrexate: a phase II, randomized, double-blind, placebo-controlled, dose-ranging trial. 2006 ACR/ARHP Annual Science Meeting; November 15. 2006. Washington DC, Presentation 2123.
45). Kay J., Matteson EL., Dasgupta B., Nash P., Durez P., Hall S, et al. Subcutaneous injection of CNTᄋ148 compared with placebo in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, dose-ranging trial. 2005 ACR/ARHP Annual Scientific Meeting; November 12-17. 2005. San Diego, CA. Presentation 1921.
46). Kaushik VV., Moots RJ. CDP-870 (certolizumab) in rheumatoid arthritis. Expert ᄋpin Biol Ther. 2005. 5:601–6.

47). Choy EH., Hazleman B., Smith M., Moss K., Lisi L., Scott DG, et al. Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology. 2002. 41:1133–7.

48). Mease P., Wei N., Fudman E., Kivitz A., Hobbs K., Anklesaria P, et al. A phase 1/2 clinical study of intra-articular administration of a recombinant adeno-associated vector containing a TNF-α antagonist gene in inflammatory arthritis. Program and abstracts of the American College of Rheumatology 70th Annual Meeting; November 1卜15. 2006. Washington, DC. Presentation 925.
49). Tutuncu ZN., Boyle D., Breitmeyer J., Kavanaugh A. AME-527, a fully human monoclonal antibody to TNF-α is well tolerated and reduces signs and symptoms of rheumatoid arthritis (RA). Program and abstracts of the American College of Rheumatology 70th Annual Meeting; November 1卜15. 2006. Washington DC, Presentation 936.
50). Hansen KE., Hildebrand JP., Genovese MC., Cush JJ., Patel S., Cooley DA, et al. The efficacy of switching from etanercept to infliximab in patients with rheumatoid arthritis. J Rheumatol. 2004. 31:1098–102.
51). Cohen G., Courvoisier N., Cohen JD., Zaltni S., Sany J., Combe B. The efficiency of switching from infliximab to etanercept and vice-versa in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2005. 23:795–800.
52). Genovese MC., Cohen S., Moreland L., Lium D., Robbins S., Newmark R, et al. (20000223 Study Group.) Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004. 50:1412–9.
53). 허민영: 신동혁: 김태환. 강직성척추염의치료. 대한류마티스학회지. 2006. 13:1–9.
54). Brown SL., Greene MH., Gershon SK., Edwards ET., Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002. 46:3151–8.

55). Setoguchi S., Solomon DH., Weinblatt ME., Katz JN., Avorn J., Glynn RJ, et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006. 54:2757–64.
56). Kremer JM. Rational use of new and existing disease-modifying agents in rheumatoid arthritis. Ann Intern Med. 2001. 134:695–706.

57). Gonzalez-Juanatey C., Testa A., Garcia-Castelo A., Garcia-Porrua C., Llorca J., Gonzalez-Gay MA. Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti-tumor necrosis factor alpha antibody. Arthritis Rheum. 2004. 51:447–50.
58). Gonzalez-Gay MA., Garcia-Unzueta MT., De Matias JM., Gonzalez-Juanatey C., Garcia-Porrua C., Sanchez-Andrade A, et al. Influence of anti-TNF-alpha infliximab therapy on adhesion molecules associated with atherogenesis in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006. 24:373–9.
Table 1.
Biological interventions investigated in rheumatoid arthritis
Table 2.
Characteristics of TNF inhibitors for treating rheumatoid arthritis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download