Abstract
Background
Induction of re-differentiation is necessary for the proper treatment of patients with recurrent or metastatic differentiated thyroid cancer (DTC) because cancer cells show de-differentiation in about 30% of these patients. In this study, we evaluated the expression of thyroid specific genes after treatment with various agents to induce re-differentiation in the follicular thyroid cancer cell line FTC-133.
Methods
FTC-133 cells were treated with U0126, LY294002, trichostatin A, retinoic acid (RA), 5’-azacytidine and α-lipoic acid (ALA). We evaluated mRNA expression of thyroid specific genes, thyroglobulin (Tg), sodium iodine symporter (NIS), PAX-8 and TTF-1 by reverse transcriptase polymerase chain reaction (PCR). Quantified expression of Tg mRNA was also evaluated by real-time PCR.
Results
The expression of Tg mRNA increased after 48 h of treatment with 0.1 uM RA and the expression of Tg mRNA and TTF-1 mRNA increased after 48–72 h of treatment with ALA (10∼100 uM). There was no change in thyroid specific gene expression by the other agents. Increased expression of Tg mRNA was confirmed by real-time PCR (1.3 times by 10 uM ALA and 3.6 times by 100 uM ALA). There was no basal NIS mRNA expression in FTC-133 cells and none of the tested agents induced expression of NIS mRNA. There was no change in phosphorylation of AMPK1-α after ALA treatment of FTC-133 cells.
Conclusion
ALA increases mRNA expression of Tg and TTF-1 of FTC-133 thyroid cancer cells and these effects are not mediated by activation of AMP kinase. The finding that ALA could be a potential re-differentiation inducing agent in thyroid cancer cells is novel. Further studies are needed to elucidate the mechanism of induction of re-differentiation. Furthermore, the effect of ALA on NIS expression and on iodine uptake should be evaluated using diverse thyroid cancer cell lines.
Go to : 
References
1. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, Mclver B, Sherman SI, Tuttle RM. American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 16:109–142. 2006.

2. Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 154:787–803. 2006.

3. Goretzki PE, Simon D, Frilling A, Witte J, Reiners C, Grussendorf M, Horster FA, Röher HD. Surgical reintervention for differentiated thyroid cancer. Br J Surg. 80:1009–1012. 1993.

4. Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M. Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 141:443–457. 1999.

5. Zarnegar R, Brunaud L, Kanauchi H, Wong M, Fung M, Ginzinger D, Duh QY, Clark OH. Increasing the effectiveness of radioactive iodine therapy in the treatment of thyroid cancer using Trichostatin A, a histone deacetylase inhibitor. Surgery. 132:984–990. 2002.

6. Van Herle AJ, Agatep ML, Padua DN 3rd, Totanes TL, Canlapan DV, Van Herle HM, Juillard GJ. Effects of 13 cis-retinoic acid on growth and differentiation of human follicular carcinoma cells (UCLA R0 82 W-1) in vitro. J Clin Endocrinol Metab. 71:755–763. 1990.
7. Schmutzler C, Winzer R, Meissner-Weigl J, Köhrle J. Retinoic acid increases sodium/iodide symporter mRNA levels in human thyroid cancer cell lines and suppresses expression of functional symporter in nontransformed FRTL-5 rat thyroid cells. Biochem Biophys Res Commun. 240:832–838. 1997.

8. Furuya F, Shimura H, Suzuki H, Taki K, Ohta K, Haraguchi K, Onaya T, Endo T, Kobayashi T. Histone deacetylase inhibitors restore radioiodide uptake and retention in poorly differentiated and anaplastic thyroid cancer cells by expression of the sodium/iodide symporter thyroperoxidase and thyroglobulin. Endocrinology. 145:2865–2875. 2004.

9. Venkataraman GM, Yatin M, Marcinek R, Ain KB. Restoration of iodide uptake in dedifferentiated thyroid carcinoma: relationship to human Na+/I-symporter gene methylation status. J Clin Endocrinol Metab. 84:2449–2457. 1999.
10. Fortunati N, Catalano MG, Arena K, Brignardello E, Piovesan A, Boccuzzi G. Valproic acid induces the expression of the Na+/I symporter and iodine uptake in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 89:1006–1009. 2004.

11. Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer. 13:797–826. 2006.

12. Liu D, Liu Z, Jiang D, Dackiw AP, Xing M. Inhibitory effects of the mitogen-activated protein kinase kinase inhibitor CI-1040 on the proliferation and tumor growth of thyroid cancer cells with BRAF or RAS mutations. J Clin Endocrinol Metab. 92:4686–4695. 2007.
13. Liu D, Hu S, Hou P, Jiang D, Condouris S, Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 13:1341–1349. 2007.

14. Kogai T, Sajid-Crockett S, Newmarch LS, Liu YY, Brent GA. Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J Endocrinol. 199:243–252. 2008.

15. Grüning T, Tiepolt C, Zöphel K, Bredow J, Kropp J, Franke WG. Retinoic acid for redifferentiation of thyroid cancer–does it hold its promise? Eur J Endocrinol. 148:395–402. 2003.
16. Courbon F, Zerdoud S, Bastie D, Archambaud F, Hoff M, Eche N, Berry I, Caron P. Defective efficacy of retinoic acid treatment in patients with metastatic thyroid carcinoma. Thyroid. 16:1025–1031. 2006.

17. Kim WG, Kim EY, Kim TY, Ryu JS, Hong SJ, Kim WB, Shong YK. Redifferentiation therapy with 13-cis retinoic acids in radioiodine-resistant thyroid cancer. Endocr J. 56:105–112. 2009.

18. Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, Haugen BR. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 93:4331–4341. 2008.

19. Novotny L, Rauko P, Cojocel C. alpha-Lipoic acid: the potential for use in cancer therapy. Neoplasma. 55:81–86. 2008.
20. Cremer DR, Rabeler R, Roberts A, Lynch B. Safety evaluation of alpha-lipoic acid (ALA). Regul Toxicol Pharmacol. 46:29–41. 2006.
21. Wenzel U, Nickel A, Daniel H. alpha-Lipoic acid induces apoptosis in human colon cancer cells by increasing mitochondrial respiration with a concomitant O2-*-generation. Apoptosis. 10:359–368. 2005.
22. Sokoloski JA, Hodnick WF, Mayne ST, Cinquina C, Kim CS, Sartorelli AC. Induction of the differentiation of HL-60 promyelocytic leukemia cells by vitamin E and other antioxidants in combination with low levels of vitamin D3: possible relationship to NF-kappaB. Leukemia. 11:1546–1553. 1997.
23. Goretzki PE, Frilling A, Simon D, Roeher HD. Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res. 118:48–63. 1990.

24. Damante G, Di Lauro R. Thyroid-specific gene expression. Biochim Biophys Acta. 1218:255–266. 1994.

25. Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 332:885–891. 2005.
26. Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 25:2488–2494. 2005.
27. Lee EY, Lee CK, Lee KU, Park JY, Cho KJ, Cho YS, Lee HR, Moon SH, Moon HB, Yoo B. Alpha-lipoic acid suppresses the development of collagen-induced arthritis and protects against bone destruction in mice. Rheumatol Int. 27:225–233. 2007.

28. Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK, Park JY, Lee KU, Kim JG, Lee IK. Alpha-lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-kappaB transcriptional activity. Exp Mol Med. 39:106–113. 2007.
29. Berkson BM, Rubin DM, Berkson AJ. The longterm survival of a patient with pancreatic cancer with metastases to the liver after treatment with the intravenous alpha-lipoic acid/low-dose naltrexone protocol. Integr Cancer Ther. 5:83–89. 2006.
30. Espinoza CR, Schmitt TL, Loos U. Thyroid transcription factor 1 and Pax8 synergistically activate the promoter of the human thyroglobulin gene. J Mol Endocrinol. 27:59–67. 2001.

31. Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. Defining thyrotropindependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci U S A. 99:15776–15781. 2002.
Go to : 
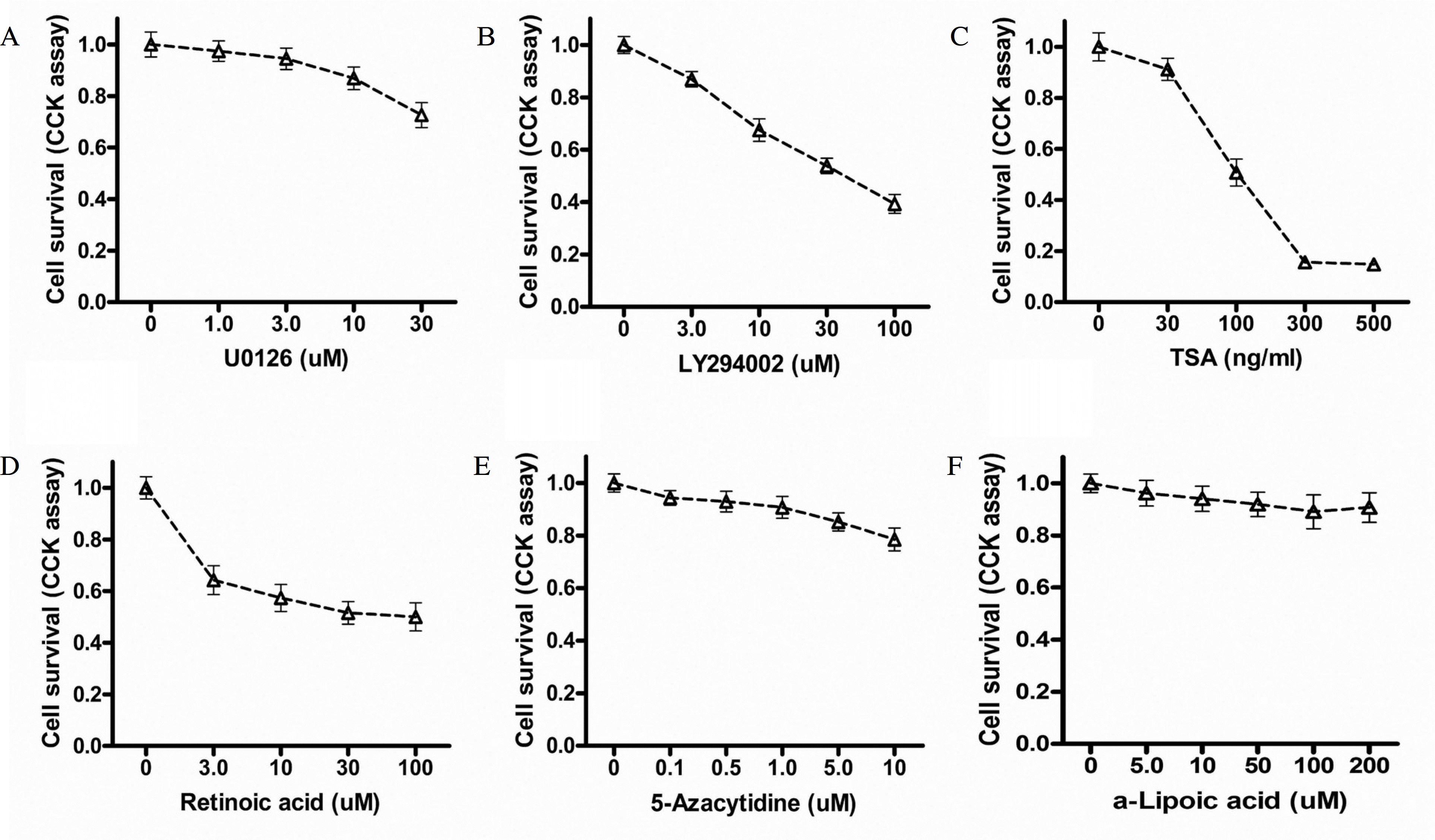 | Fig. 1.Cell survival curves measured by cell counting kit-8 after 48 hours of treatment with re-differentiation agents in FTC-133 cells. U0126; selective inhibitor of MEK1/2, LY294002; selective inhibitor of PI3K, TSA; trichostatin A. U0126 (A), LY294002 (B), TSA (C), Retinoic acid (D), 5-Azacytidine (E), a-Lipoic acid (F). |
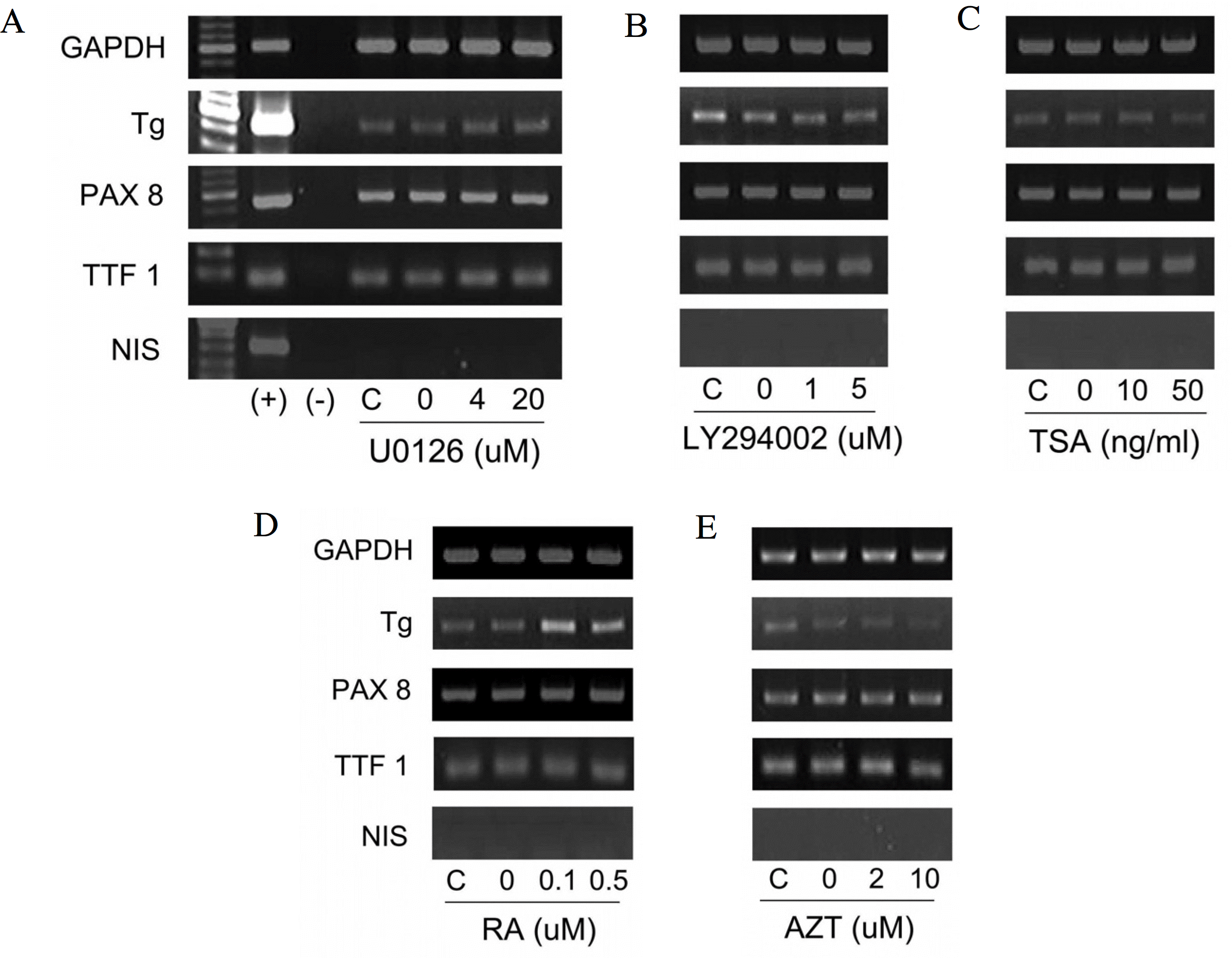 | Fig. 2.Results of reverse transcriptase polymerase chain reaction after 48 hours of treatment with re-differentiation agents in FTC-133 cells. GAPDH, glyceraldehydes 3-phosphate dehydrogenase; Tg, thyroglobulin; PAX-8, paired box 8; TTF-1, thyroid transcription factor-1; NIS, sodium iodine symporter; (+), positive control (human thyroid tissue); (−), negative control (no template); C, control without vehicle; U0126, selective inhibitor of MEK1/2; LY294002, selective inhibitor of PI3K; TSA, trichostatin A; RA, retinoic acid; AZT, 5’-azacytidine. U0126 (A), LY294002 (B), TSA (C), RA (D), AZT (E). |
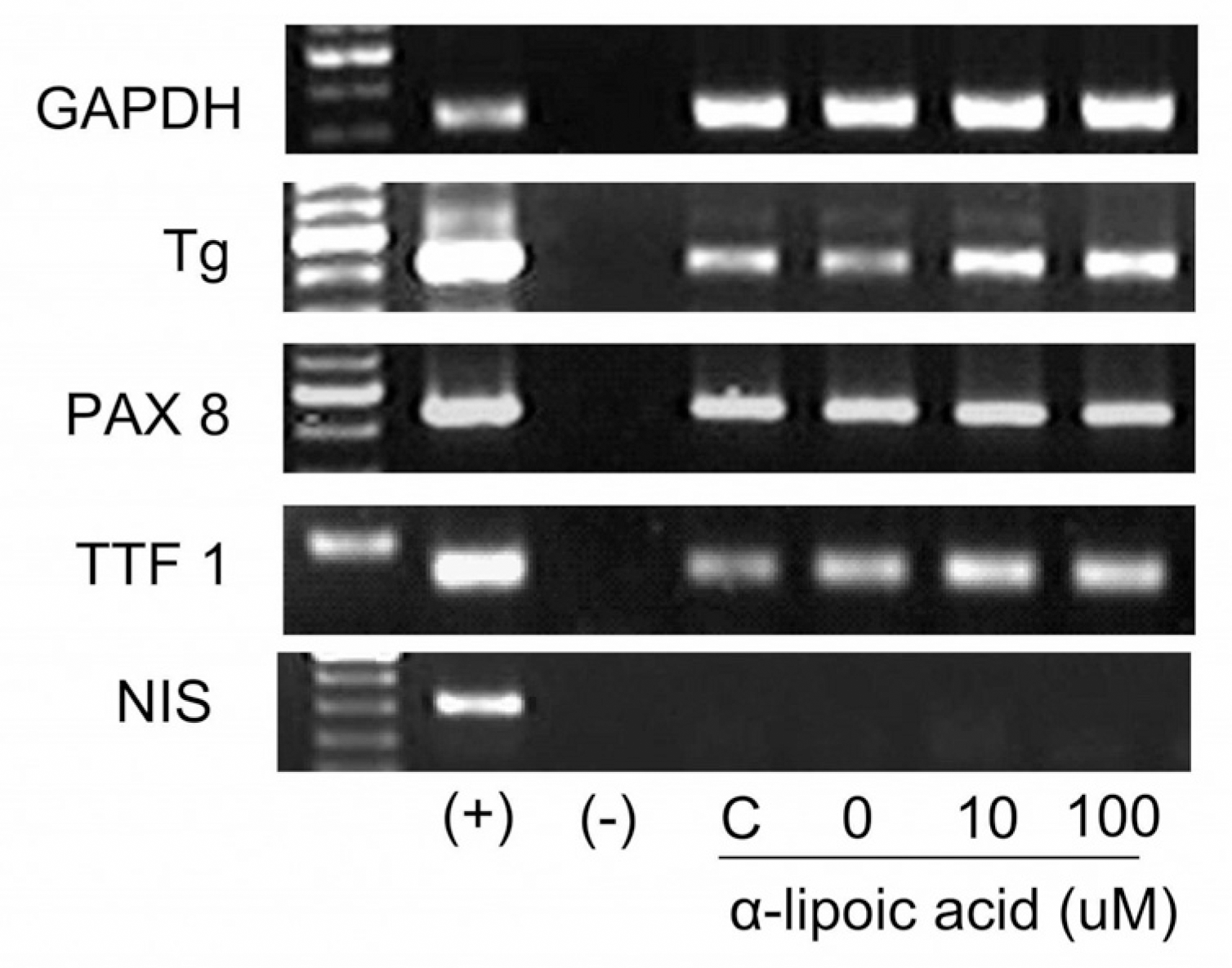 | Fig. 3.Results of reverse transcriptase polymerase chain reaction after 72 hours of treatment with α-lipoic acids in FTC-133 cells. GAPDH, glyceraldehydes 3-phosphate dehydrogenase; Tg, thyroglobulin; PAX-8, paired box 8; TTF-1, thyroid transcription factor-1; NIS, sodium iodine symporter; (+), positive control (human thyroid tissue); (−), negative control (no template); C, control without vehicle. |
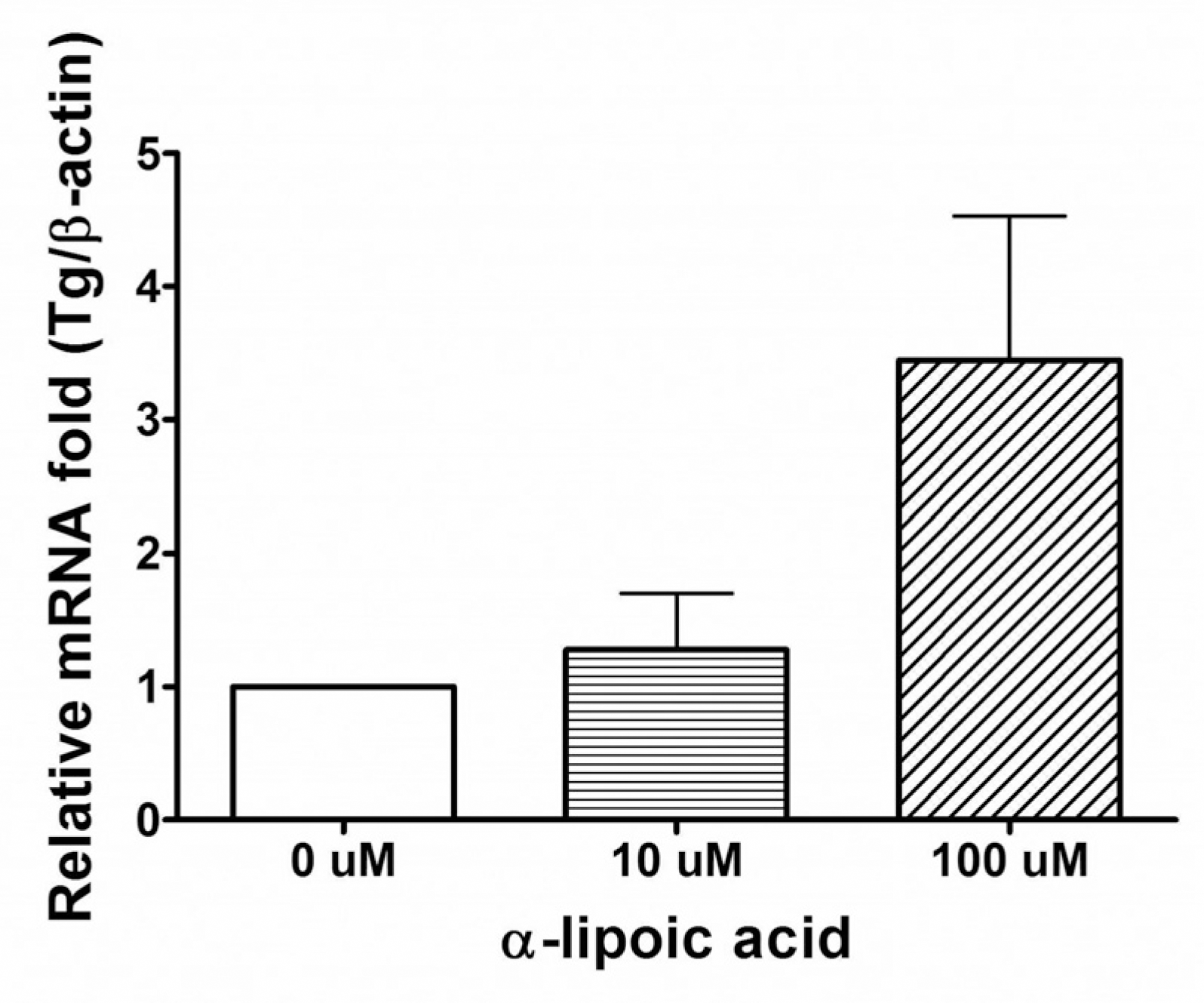 | Fig. 4.Results of real-time polymerase chain reaction after 72 hours of treatment with α-lipoic acids in FTC-133 cells. |
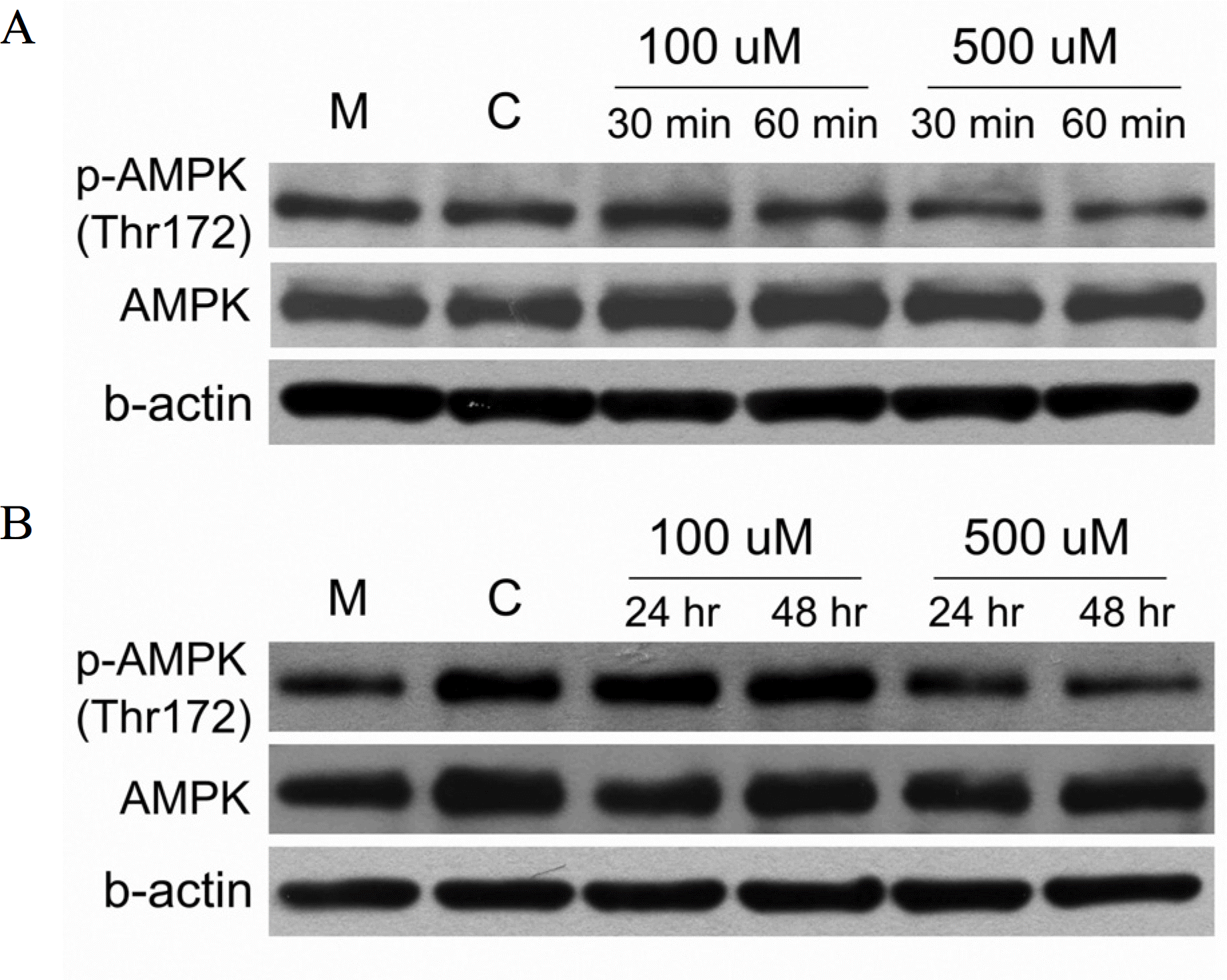 | Fig. 5.Western blotting of phospho-5-AMP-activated protein kinase (AMPK)-1α and total-AMPK1-α protein after treatment of α-lipoic acids during 30∼60 minutes (A) and 24–48 hours (B) in FTC-133 cells. |
Table 1.
Primers and conditions of reverse transcriptase polymerase chain reaction for thyroid specific genes in FTC-133 cell




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download