Abstract
Background
Peroxisome proliferator-activated receptor delta (PPAR-δ) is a ligand-activated nuclear transcription factor that is associated with many diseases, such as diabetes, obesity, metabolic syndrome, and cancer. However, the function of PPAR-δ is controversial in carcinogenesis since its ligands may inhibit or promote the growth of cancer cells. The purpose of this study was to determine the effect of GW501516, the specific agonist of PPAR-δ, in the growth and invasiveness of thyroid cancer cell lines by modulation of the target genes, ANGPTL-4 and MCP-1.
Methods
Three kinds of human cancer cell lines, FRO (thyroid anaplastic carcinoma), NPA (melanoma), and ARO (colon cancer) were treated with GW501516 in serum-free media. Cell viability was assayed using a colorimetric cell counting kit-8 assay. The changes in the level of expression of PPAR-δ and its target genes, angiopoietin-like protein-4 (ANGPTL-4) and monocyte chemotactic protein-1 (MCP-1), were determined by RT-PCR analysis and invasiveness was assessed by a cell invasion assay kit.
Results
GW501516 inhibited the cell growth of cancer cell lines in a dose-dependent manner and modulated the stimulation of ANGPTL-4, as well as inhibition of MCP-1. These effects were more prominent in NPA and ARO, but less effective in the thyroid cancer cell line, which had higher PPAR-δ and lower ANGPTL-4 mRNA levels. The inhibitory effects of GW501516 on cancer invasiveness had a similar pattern.
Conclusion
The activation of PPAR-δ by GW501516 reduced the cell growth and invasiveness of the thyroid cancer cell line. This effect of GW501516 was associated with a stimulatory effect of ANGPTL4 and an inhibitory effect of MCP-1 in cancer cell lines. GW501516 was less effective in the thyroid cancer cell line, which had a low basal ANGPTL-4 mRNA level. The findings of our study serve as an impetus for further studies to elucidate the precise role of ANGPTL-4 and PPAR-δ in carcinogenesis.
Figures and Tables
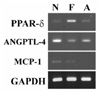 | Fig. 1Expression of the PPAR-δ and its target genes, ANGPTL-4 and MCP-1 in human cancer cell lines. Total RNA was isolated from cancer cell lines, ARO, FRO and NPA. The expression of PPARδ, ANGPTL-4, and MCP-1 gene were determined by RT-PCR analysis. N, NPA; F, FRO; A, ARO |
 | Fig. 2Effect of GW501516 on cell viability in human cancer cell lines, NPA, ARO, and FRO. Cells were exposed to the indicated concentrations of GW501516 for 48 hours and cell viability was determined by CCK assay. Data presented are mean ± SD for three independent experiments. |
 | Fig. 4Effect of GW501516 on PPAR-δ and its target genes, ANGPTL4 and MCP-1 expression. Cells were exposed to 10 uM GW501516 for 0~48 hr. and the change of PPAR-δ, ANGPTL-4, and MCP-1 expressions were determined by RT-PCR analysis. Expression of GAPDH was used as loading controls. A. NPA. B. ARO. C. FRO cells. |
References
1. Sherman SI. Thyroid carcinoma. Lancet. 2003. 361:501–511.
2. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006. 295:2164–2167.
3. Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in france: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004. 14:1056–1060.
4. Lubina A, Cohen O, Barchana M, Liphshiz I, Vered I, Sadetzki S, Karasik A. Time trends of incidence rates of thyroid cancer in Israel: what might explain the sharp increase. Thyroid. 2006. 16:1033–1040.
5. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006. 6:292–306.
6. Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003. 113:159–170.
7. Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006. 116:581–589.
8. Tsubouchi Y, Sano H, Kawahito Y, Mukai S, Yamada R, Kohno M, Inoue K, Hla T, Kondo M. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-gamma agonists through induction of apoptosis. Biochem Biophys Res Commun. 2000. 270:400–405.
9. Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta. 2007. 1771:972–982.
10. Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 1998. 139:2748–2754.
11. Petrashevskaya NN, Schwarz A. Peroxisome proliferator-activated receptor beta/delta: a new antihypertrophic drug target? Cardiovasc Res. 2005. 65:770–771.
12. Burdick AD, Bility MT, Girroir EE, Billin AN, Willson TM, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-beta/delta(PPARbeta/delta) inhibits cell growth of human N/TERT-1 keratinocytes. Cell Signal. 2007. 19:1163–1171.
13. Xu L, Han C, Wu T. A novel positive feedback loop between peroxisome proliferator-activated receptor-delta and prostaglandin E2 signaling pathways for human cholangiocarcinoma cell growth. J Biol Chem. 2006. 281:33982–33996.
14. Hollingshead HE, Killins RL, Borland MG, Girroir EE, Billin AN, Willson TM, Sharma AK, Amin S, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) ligands do not potentiate growth of human cancer cell lines. Carcinogenesis. 2007. 28:2641–2649.
15. Marin HE, Peraza MA, Billin AN, Willson TM, Ward JM, Kennett MJ, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor beta inhibits colon carcinogenesis. Cancer Res. 2006. 66:4394–4401.
16. Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med. 2004. 10:245–247.
17. Stephen RL, Gustafsson MC, Jarvis M, Tatoud R, Marshall BR, Knight D, Ehrenborg E, Harris AL, Wolf CR, Palmer CN. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004. 64:3162–3170.
18. Girroir EE, Hollingshead HE, Billin AN, Willson TM, Robertson GP, Sharma AK, Amin S, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) ligands inhibit growth of UACC903 and MCF7 human cancer cell lines. Toxicology. 2008. 243:236–243.
19. Oliver WR Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001. 98:5306–5311.
20. Chen LC, Hao CY, Chiu YS, Wong P, Melnick JS, Brotman M, Moretto J, Mendes F, Smith AP, Bennington JL, Moore D, Lee NM. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004. 64:3694–3700.
21. Wang D, Wang H, Guo Y, Ning W, Katkuri S, Wahli W, Desvergne B, Dey SK, DuBois RN. Crosstalk between peroxisome proliferator-activated receptor delta and VEGF stimulates cancer progression. Proc Natl Acad Sci U S A. 2006. 103:19069–19074.
22. Fukumoto K, Yano Y, Virgona N, Hagiwara H, Sato H, Senba H, Suzuki K, Asano R, Yamada K, Yano T. Peroxisome proliferator-activated receptor delta as a molecular target to regulate lung cancer cell growth. FEBS Lett. 2005. 579:3829–3836.
23. Galaup A, Cazes A, Le Jan S, Philippe J, Connault E, Le Coz E, Mekid H, Mir LM, Opolon P, Corvol P, Monnot C, Germain S. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci U S A. 2006. 103:18721–18726.
24. Cazes A, Galaup A, Chomel C, Bignon M, Brechot N, Le Jan S, Weber H, Corvol P, Muller L, Germain S, Monnot C. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ Res. 2006. 99:1207–1215.
25. Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000. 275:28488–28493.
26. Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol. 2000. 20:5343–5349.
27. Staiger H, Haas C, Machann J, Werner R, Weisser M, Schick F, Machicao F, Stefan N, Fritsche A, Haring HU. Muscle-Derived Angiopoietin-Like Protein 4 is Induced by Fatty Acids via PPAR{delta} and is of Metabolic Relevance in Humans. Diabetes. 2008.
28. Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, Tsuchihashi M, Sugaya T, Charo IF, Kura S, Tsuzuki T, Ishibashi T, Takeshita A, Egashira K. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004. 94:1203–1210.
29. Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J Biol Chem. 2008. 283:14542–14551.
30. Negus RP, Stamp GW, Relf MG, Burke F, Malik ST, Bernasconi S, Allavena P, Sozzani S, Mantovani A, Balkwill FR. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995. 95:2391–2396.
31. Kuroda T, Kitadai Y, Tanaka S, Yang X, Mukaida N, Yoshihara M, Chayama K. Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res. 2005. 11:7629–7636.
32. Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer. 2002. 102:220–224.
33. Ghilardi G, Biondi ML, La Torre A, Battaglioli L, Scorza R. Breast cancer progression and host polymorphisms in the chemokine system: role of the macrophage chemoattractant protein-1 (MCP-1) -2518 G allele. Clin Chem. 2005. 51:452–455.
34. Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003. 302:453–457.
35. Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, Yamazaki Y, Kuroda J, Shibata N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006. 536:182–191.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download