References
1. Weinstein RS. Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disord. 2:65–73. 2001.
2. Kim HJ, Zhao H, Kitaura H, Bhattacharyya S, Brewer JA, Muglia LJ, Ross FP, Teitelbaum SL. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 116:2152–2160. 2006.
3. Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 109:1041–1048. 2002.

4. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 102:274–282. 1998.

5. Liu Y, Porta A, Peng X, Gengaro K, Cunningham EB, Li H, Dominguez LA, Bellido T, Christakos S. Prevention of glucocorticoid-induced apoptosis in osteocytes and osteoblasts by calbindin-D28k. J Bone Miner Res. 19:479–490. 2004.

6. Smith E, Coetzee GA, Frenkel B. Glucocorticoids inhibit cell cycle progression in differentiating osteoblasts via glycogen synthase kinase-3beta. J Biol Chem. 277:18191–18197. 2002.
7. Canalis E. Clinical review 83: Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 81:3441–3447. 1996.

8. Pereira RC, Delany AM, Canalis E. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone. 30:685–691. 2002.

9. Pereira RM, Delany AM, Canalis E. Cortisol inhibits the differentiation and apoptosis of osteoblasts in culture. Bone. 28:484–490. 2001.

10. Ito S, Suzuki N, Kato S, Takahashi T, Takagi M. Glucocorticoids induce the differentiation of a mesenchymal progenitor cell line, ROB-C26 into adipocytes and osteoblasts, but fail to induce terminal osteoblast differentiation. Bone. 40:84–92. 2007.

11. Pereira RC, Delany AM, Canalis E. CCAAT/enhancer binding protein homologous protein (DDIT3) induces osteoblastic cell differentiation. Endocrinology. 145:1952–1960. 2004.

13. Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 16:4128–4136. 1996.

14. Wedel A, Ziegler-Heitbrock HW. The C/EBP family of transcription factors. Immunobiology. 193:171–185. 1995.

15. Ohnaka K, Tanabe M, Kawate H, Nawata H, Takayanagi R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun. 329:177–181. 2005.

16. Smith E, Frenkel B. Glucocorticoids inhibit the transcriptional activity of LEF/TCF in differentiating osteoblasts in a glycogen synthase kinase-3beta-dependent and -independent manner. J Biol Chem. 280:2388–2394. 2005.
17. Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem. 72:396–410. 1999.

18. Purpura KA, Aubin JE, Zandstra PW. Sustained in vitro expansion of bone progenitors is cell density dependent. Stem Cells. 22:39–50. 2004.

19. Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 140:4382–4389. 1999.
20. Rubin J, Biskobing DM, Jadhav L, Fan D, Nanes MS, Perkins S, Fan X. Dexamethasone promotes expression of membrane-bound macrophage colony-stimulating factor in murine osteoblast-like cells. Endocrinology. 139:1006–1012. 1998.
21. Pearce G, Tabensky DA, Delmas PD, Baker HW, Seeman E. Corticosteroid-induced bone loss in men. J Clin Endocrinol Metab. 83:801–806. 1998.

22. Prummel MF, Wiersinga WM, Lips P, Sanders GT, Sauerwein HP. The course of biochemical parameters of bone turnover during treatment with corticosteroids. J Clin Endocrinol Metab. 72:382–386. 1991.

23. Dempster DW. Bone histomorphometry in glucocorticoid-induced osteoporosis. J Bone Miner Res. 4:137–141. 1989.
24. Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 349:1207–1215. 2003.

25. Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 349:1216–1226. 2003.

26. Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 170:427–435. 2007.

27. Faccio R, Novack DV, Zallone A, Ross FP, Teitelbaum SL. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin. J Cell Biol. 162:499–509. 2003.
28. Nakamura I, Kadono Y, Takayanagi H, Jimi E, Miyazaki T, Oda H, Nakamura K, Tanaka S, Rodan GA, Duong le T. IL-1 regulates cytoskeletal organization in osteoclasts via TNF receptor-associated factor 6/c-Src complex. J Immunol. 168:5103–5109. 2002.

29. Chellaiah MA, Soga N, Swanson S, McAllister S, Alvarez U, Wang D, Dowdy SF, Hruska KA. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J Biol Chem. 275:11993–12002. 2000.

30. Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 11:284–290. 2005.

31. Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 12:46–54. 2002.

33. Marzia M, Chiusaroli R, Neff L, Kim NY, Chishti AH, Baron R, Horne WC. Calpain is required for normal osteoclast function and is downregulated by calcitonin. J Biol Chem. 281:9745–9754. 2006.

34. Kim H-J, Hong J-M, Kim T-H, Ross FP, Teitelbaum SL, Kim S-Y. Calpain 6 regulates osteclastic bone resorption via cytoskeletal organizaion and microtubule acetylation. (unpublished).
35. Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 14:407–416. 2003.

36. McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 105:433–440. 2000.
Fig. 1.
Regulation of osteoclast formation and function. Osteoclasts are derived from hematopoietic mononuclear precursors of the monocyte/macrophage lineage. The proliferation of osteoclast precursors is dependent on the M-CSF. Activation of RANK by RANKL (RANK-Ligand) commits the cell to the osteoclast fate. The initial event in development of the resorptive capacity of the mature osteoclast is its cytoskeletal organization, namely polarization. Once polarized, the osteoclasts resorb the mineralized component of bone.
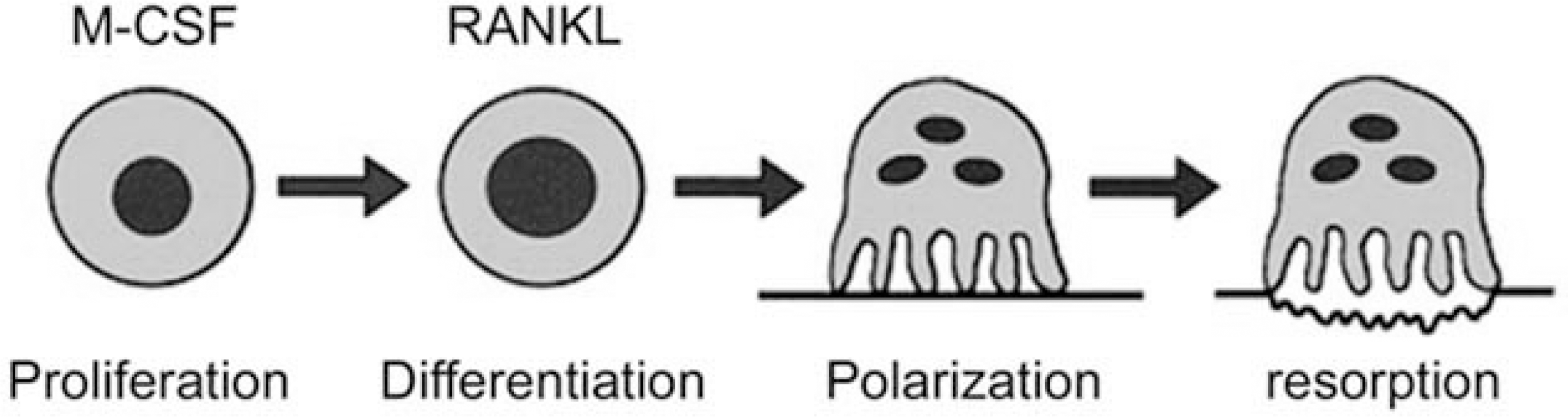
Fig. 2.
Capn6 promotes cytoskeletal organization, microtubule stability, and the expression of β3 integrin protein in osteoclasts and its inhibition may be a means by which glucocorticoids suppress bone remodeling.
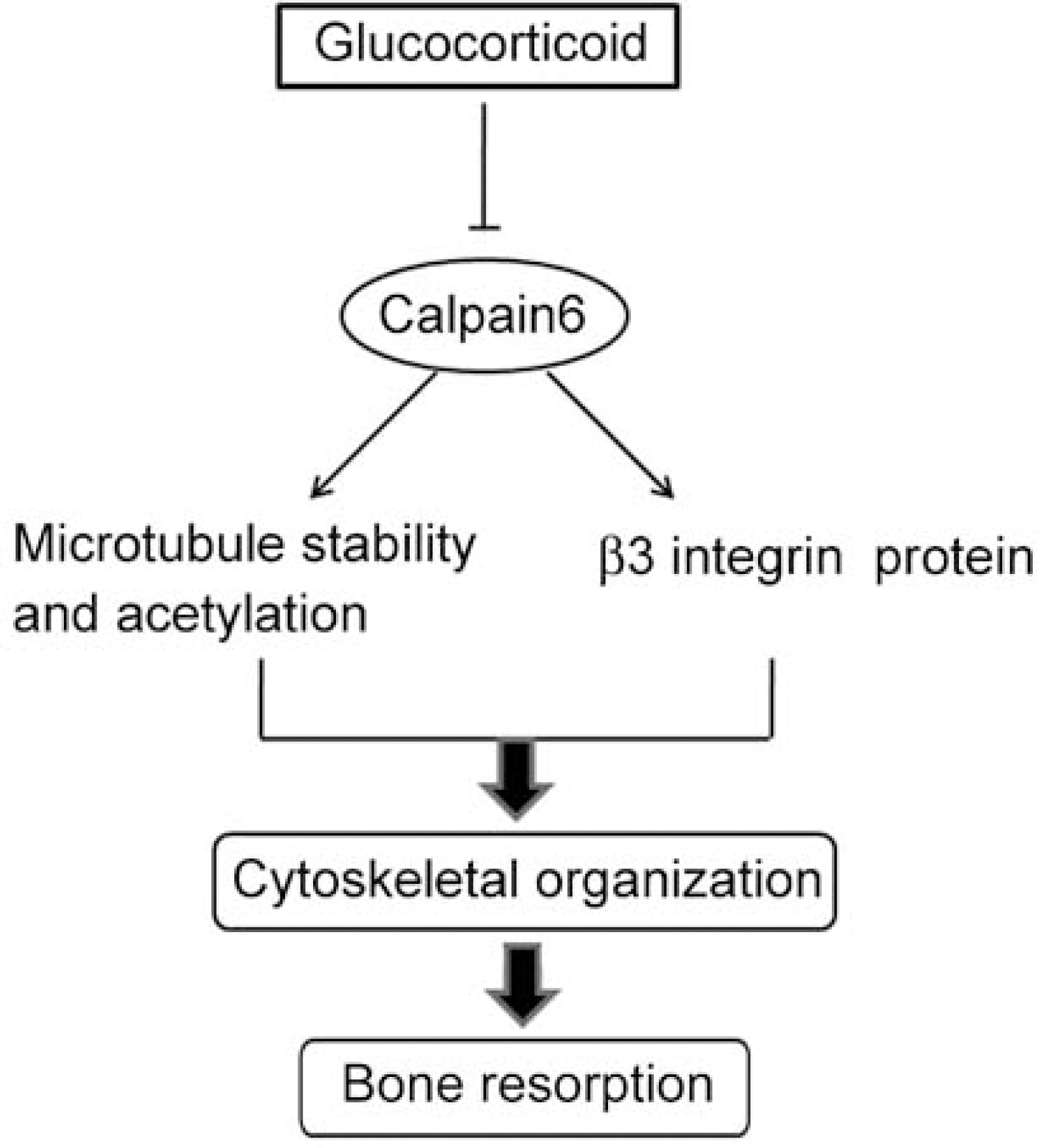
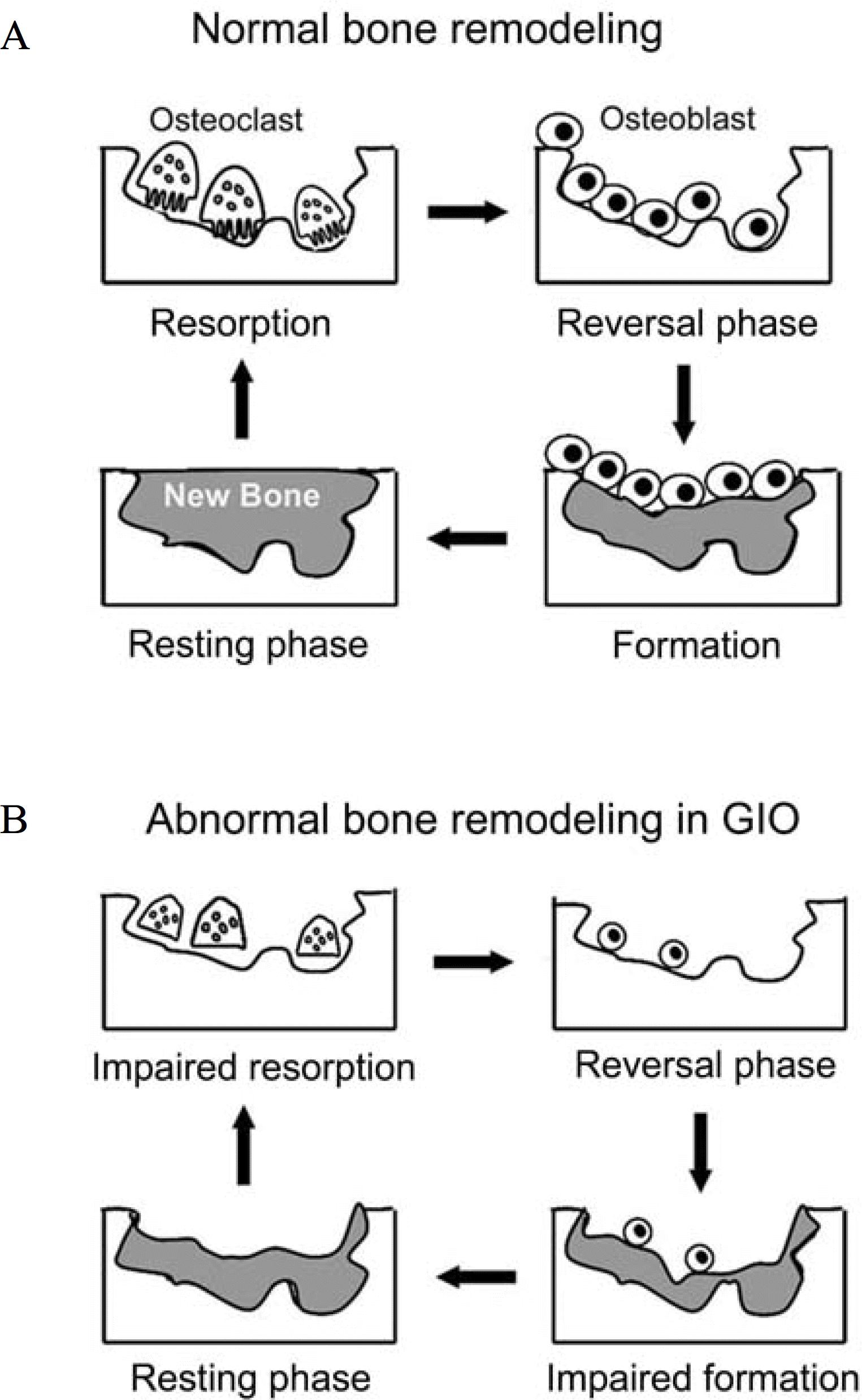
Fig. 3.
Comparison of a normal cycle of bone remodeling (A) with an abnormal one caused by glucocorticoid excess (B). Resorptive phase: activated osteoclasts resorb a discrete area of mineralized bone matrix. Reversal phase: subsequently osteoblasts migrate into resorption lacuna, possibly by factors produced by the osteoclast. Formative phase: the osteoblasts deposit new bone matrix, possibly by factors produced by the osteoclast or released from the bone matrix. Resting phase: once embedded, the osteoblasts mature into terminally differentiated osteocytes. Note the impaired recruitment and decreased number of osteoblast as well as the incomplete repair of bone in glucocoticoid-induced osteoporosis (GIO). Newly formed bone is in gray.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download