Introduction
Intramural hematoma of the aorta is a variant of aortic dissection characterized by the absence of direct communication between the false lumen and the true lumen of the aorta[1]. While aortic intramural hematoma is similar to aortic dissection in many aspects of clinical manifestations, the underlying pathophysiology of two diseases may differ. Intramural hematoma may result from the rupture of the vasa vasorum, which cause subsequent extension of subintimal hemorrhage. The subsequently weakened aortic wall becomes less distensible and may develop complication such as outward wall rupture or inward intimal disruption[1].
Primary aldosteronism is associated with arterial hypertension. Aldosterone in primary aldosteronism autonomously secrete in part, and the plasma renin activity is suppressed[2]. Primary aldosteronism may cause direct alterations in arterial structure through the pleiotropic effects of aldosterone, as well as indirect alterations through pressure effects[3]. There have been several reported cases of aortic dissection in patients with primary aldosteronism, which suggests a causal relationship between the two diagnostic entities[4~5]. However, intramural hematoma formation has not been described in patients with primary aldosteronism.
Here, we describe an aortic intramural hematoma in a patient with primary aldosteronism and speculate about the causal relationship between these two entities.
Case
A 48-year-old woman presented to the hospital with severe upper back pain. Hypertension had been detected 1 year earlier at another facility. She was taking lercanidipine (20 mg/day) and fosinopril (20 mg/day) for her hypertension, which was relatively well controlled. She did not smoke or drink, and she had no family history of endocrine disorders.
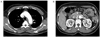
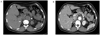
Upon admission, she had a body temperature of 36.5℃, a heart rate of 114 beats/min, and a blood pressure of 190/110 mmHg. Physical examination was unremarkable. Electrocardiography showed no abnormalities. On laboratory examination, the serum potassium and serum creatinine levels were 2.6 mEq/L and 0.4 mg/dL, respectively, and other biochemistries were all within normal range. Transthoracic echocardiogram revealed a normal left ventricular chamber size with good left ventricular systolic function (ejection fraction 73%) without regional wall motion abnormalities. The patient had no visible abnormal flap and no aortic insufficiency. Computerized tomography of the thorax and the abdomen showed an intramural hematoma beginning at the aortic arch and extending into the infrarenal abdominal aorta, without a flap (Fig. 1). There was a 1.1 × 1.1 cm sized nodular lesion in the right adrenal gland, but the left adrenal gland was unremarkable (Fig. 2). The patient was started on an aggressive antihypertensive regimen for the management of her intramural hematoma. She was evaluated for an adrenal mass. The morning plasma renin activity and aldosterone level were 0.2 ng/mL/h and 22.1 ng/dL, respectively. The plasma aldosterone concentration to plasma renin activity ratio (ARR) was 110.5. The 24-hour urine free cortisol, vanillylmandelic acid, and metanephrine were all normal. A diagnosis of primary aldosteronism was suspected. After fosinopril was hold for 2 weeks, the repeat renin activity and aldosterone level were 0.14 ng/mL/h and 32.5 ng/dL, respectively, with an ARR of 232.1. To confirm primary aldosteronism, the patient was given 50 mg of PO captopril. After the captopril test, she had an ARR of 93.4 (cut-off value ≥ 30). She was diagnosed with an intramural hematoma of the aorta in the setting of primary aldosteronism, and her antihypertensive regimen was modified to include spironolactone. Normokalemia was maintained without a potassium supplement. The patient refused surgical resection of the adrenal adenoma and decided to continue medical management with serial follow-up.
Discussion
Aortic dissection is characterized by the occurrence of an intimal flap, which separate the true and false lumens. The incidence of this clinical condition is 5~30 cases per one million people per year[6]. However, intramural hematoma of the aorta is characterized by the absence of direct communication between the false lumen and the true lumen. Intramural hematomas occur in 4% to 27% of patients with suspected aortic dissection[1,7]. Although it is a controversial suggestion, there are several differences between intramural hematomas and aortic dissections that may suggest the two are distinct entities. Intramural hematomas tend to be associated with more advanced age at onset[8], a lower frequency of malperfusion syndrome[8], localization in the descending aorta[9] and hematoma formation within the media closer to the adventitia, as well as medial degeneration with elastin fragmentation and smooth muscle cell loss[10].
Intramural hematoma formation may result from the rupture of either diseased or non-diseased vasa vasorum without support of the surrounding media[7]. Primary aldosteronism, which is an uncommon cause of hypertension with an incidence of less than 1%[2], may be a risk factor for intramural hematoma formation independent of hypertension. The classical target of aldosterone is the distal convoluted tubule of the kidney, where it modulates sodium, potassium, and body fluid balance. Furthermore, as mineralocorticoid receptors are expressed in the heart and blood vessels, aldosterone may directly mediate its detrimental effects throughout the entire cardiovascular system[3]. Excessive stimulation of these receptors may break the balance in the antioxidant-oxidant milieu, which causes endothelial dysfunction, left ventricular hypertrophy, and nephrosclerosis with perivascular fibrosis of the atria, both ventricles, and aorta[3]. Aldosterone may affect the matrix metalloproteinases and their inhibitors, leading to collagen degradation and subsequent medial necrosis[4]. Aldosterone may activate the adventitia, resulting in proliferation and migration of fibroblast and secretion of collagen to exacerbate vascular remodeling[11]. Fibrosis of the adventitia may cause the obstruction of vessels that feed the small intramural vasa vasorum, and reduction of nutritional supply to the media may result in necrosis of the smooth muscle cells[12]. These effects may culminate in the development of cardiovascular complications such as intramural hematoma formation and aortic dissection[13]. In the present case, the relatively young age of the patient suggested the possibility that the aldosterone hypersecretion contributed to the vascular damage.
The hallmark of primary aldosteronism is suppressed plasma renin activity and elevated aldosterone. One can screen for primary aldosteronism by measuring the plasma aldosterone to plasma renin activity ratio[2]. The captopril test has been used for the purpose of either screening or confirmation[2]. In patients with primary hypertension, acute angiotensin converting enzyme (ACE) inhibition decreases aldosterone production mediated by rennin-angiotensin system. However, this decrease does not occur in primary aldosteronism, reflecting the autonomy of aldosterone production. An ARR of 30 after administration of captopril suggests the presence of primary aldosteronism[14]. In the present case, the patient underwent the captopril test so volume expansion could be avoided through sodium loading or fludrocortisone administration. The patient had a high ARR, confirming the diagnosis of primary aldosteronism.
Operative correction is required in the vast majority of ascending aorta intramural hematomas, though intramural hematomas of the descending thoracic aorta can be managed medically[7]. The treatment for an aldosterone-producing adenoma is adrenalectomy[2]. However, medical therapy with drugs that block aldosterone is a viable option for controlling blood pressure and serum potassium concentration levels[15~17].
Our case suggests that primary aldosteronism should always be considered in patients with secondary hypertension to avoid the adverse effects of aldosterone excess on the cardiovascular system, and clinicians should screen for primary aldosteronism in patients with intramural hematomas.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download