1. Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB Jr, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 54:3140–3147. 2005.
2. Mulhall BP, Ong JP, Younossi ZM. Non-alcoholic fatty liver disease: an overview. J Gastroenterol Hepatol. 17:1136–1143. 2002.

3. Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol 17 (suppl): S186-S190,. 2002.
4. Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 98:960–967. 2003.

5. Sattar N, Scherbakova O, Ford I, O'Reilly DS, Stanley A, Forrest E, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Elevated alanine aminotranferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the West of Scotland Coronary Prevaention Study. Diabetes. 53:2855–2860. 2004.
6. Nakanishi N, Suzuke K, Tatara K. Serum r-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 27:1427–1432. 2004.
7. Cho NH, Lee HK, Jang HC, Chan JCN, Choi SH, Lim S, Kim HR. Abnormal liver function test predicts type 2 diabetes. Diabetes Care. 30:2566–2568. 2007.

8. Lee DH, Steffes MW, Jacobs DR Jr. Can persistent organic pollutants explain the association between serum gamma-glutamyltransferase and type 2 diabetes? Diabetologia. 51:402–407. 2008.
9. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostatic model assessment: insulin resistance and b-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 28:412–419. 1985.
10. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 285:2486–2496. 2001.
11. Chung HW, Kim DJ, Jin HD, Choi SH, Ahn CW, Cha BS, Lee HC, Huh KB. Prevalence of metabolic syndrome according to the new criteria for obesity. J Kor Diabetes Assoc. 26:431–442. 2002.
12. Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care. 25:1612–1628. 2002.

13. Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, Kim NH, Choi DS, Baik SH. Serum adiponectin concentrations predict the developments of type 2 diabetes and the metabolic syndrome in elderly Koreans. Clin Endocrinol. 61:75–80. 2004.

14. Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 25:2016–2021. 2002.

15. Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Hepatic enzyme, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care. 28:2913–2918. 2005.
16. Andre P, Balkan B, Vol S, Charles MA. Eschweqe E DESIR study group. Gamma-glutamyltransferase activity and development of the metabolic syndrome (International Diabetes Federation Definition) in middle-aged man and women, data from the Epidemiological Study on the Insulin Resistance Syndrome(DESIR) cohort. Diabetes Care. 30:2355–2361. 2007.
17. Marchesini G, Brisi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 50:1844–1850. 2001.
18. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 37:917–923. 2003.
19. Liangpunsakul S, Chalasani N. Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: results from the third National Health and Nutrition Survey (NHANES III). Am J Med Sci. 329:111–116. 2005.

20. Browning JD, Szezepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 40:1387–1395. 2004.

21. Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 87:3023–3028. 2002.

22. Tiikkainen M, Bergholm R, Vehkavaara S, Rissanen A, Hakkinen AM, Tamminen M, Teramo K, Yki-Jarvinen H. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 52:701–707. 2003.

23. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 342:1266–1271. 2000.

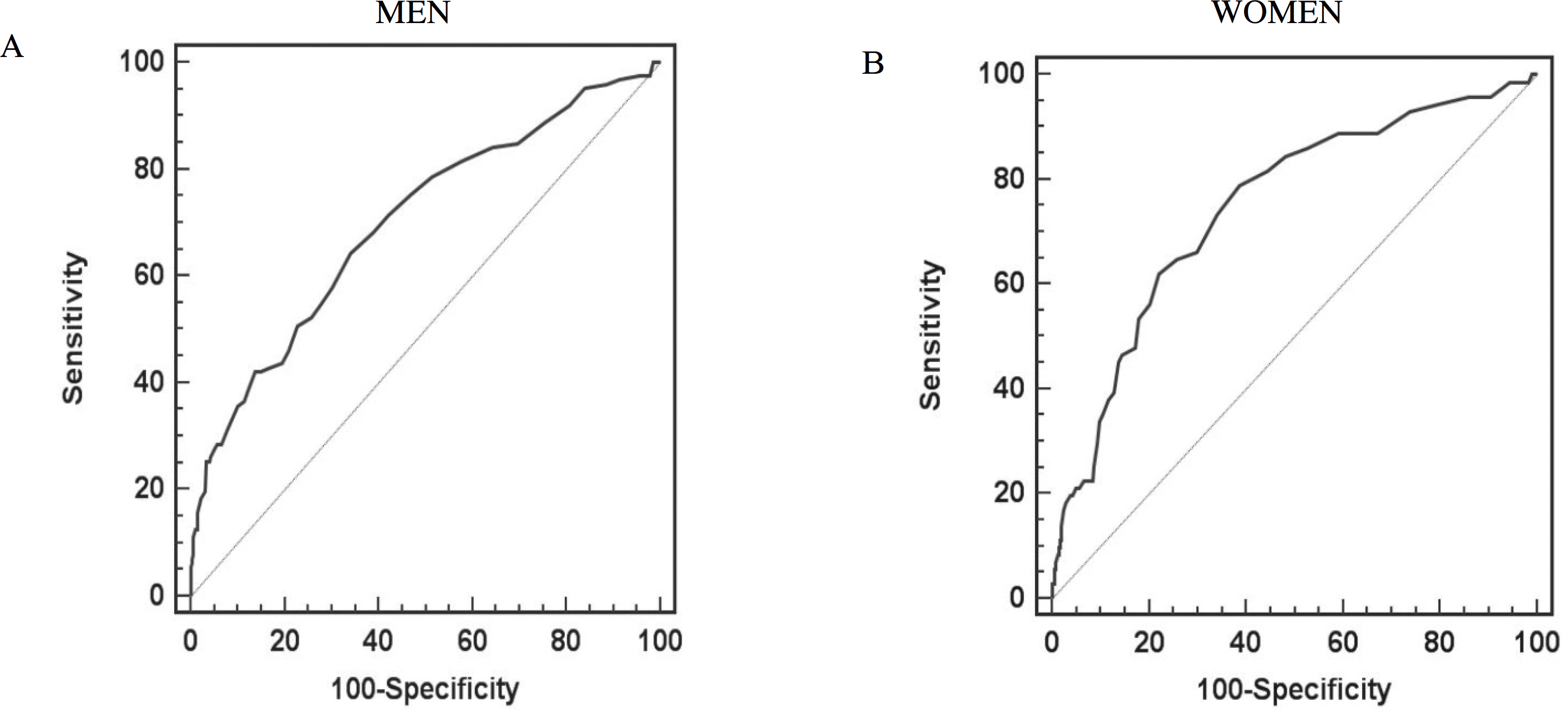
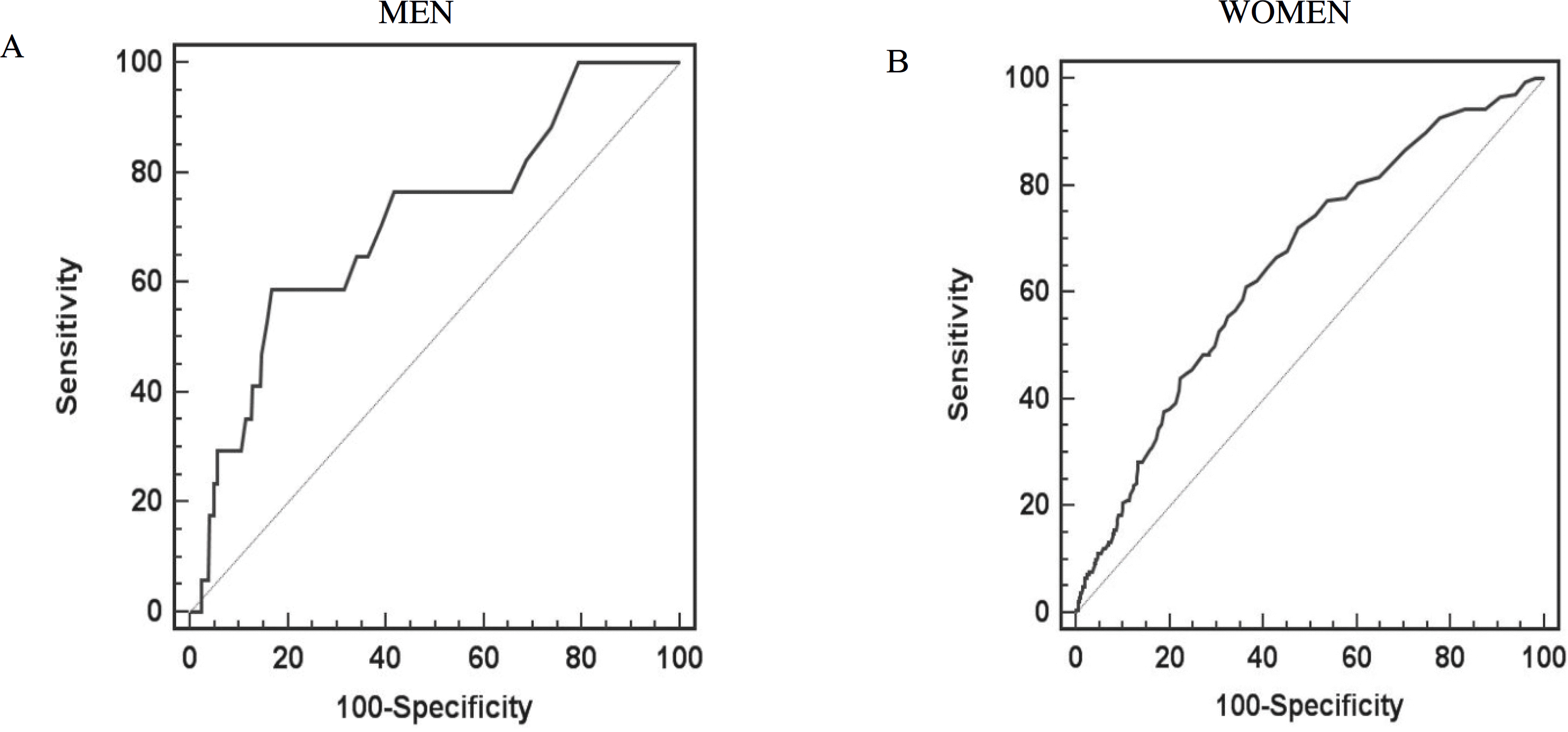




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download