Abstract
Purpose
Tumor cell invasion is characteristic of malignant neoplasms. S100A4, a member of a family of small calcium binding proteins, and COX2, seem to have a role in promoting progression and invasion of many human cancers. The clinical stage of a papillary thyroid carcinoma (PTC) depends on age, tumor size, and extrathyroidal extension. Extrathyroidal extension is correlated with the invasiveness of a tumor. However, there are no reliable prediction markers for invasiveness. We evaluated S100A4 and COX2 expression in PTCs to determine if expression correlates with invasiveness, and if expression of S100A4 and COX2 are useful as prediction markers.
Methods
The expression of S100A4 and COX2 were evaluated using immunohistochemical analysis in 35 PTC specimens.
Results
More intense staining in cells that invaded the front portion rather than the central portion of a PTC was indicative of increased expression of S100A4 and COX2. Therefore, cases were analyzed for extent of staining in tumor cells that invaded the front portion of a PTC. High expression group (higher expression than average expression rate) of S100A4 and COX2 were significantly correlated with extrathyroidal extension (P = 0.0094 and P = 0.0433, respectively). However, no other clinicopathological factors including age, lymph node involvement, and multiplicity were related to expression of S100A4 and COX2, as determined in this study.
References
1. Siironen P, Louhimo J, Nordling S, Ristimaki A, Maenpaa H, Haapiainen R, Caj H. Prognostic factors in papillary thyroid cancer: an evaluation of 601 consecutive patients. Tumor Biol. 26:57–64. 2005.

2. Barraclough R. Calcium-binding protein S100A4 in health and disease. Biochimica et Biophysica Acta. 1448:190–199. 1998.

3. Ito Y, Yoshida H, Tomoda C, Uruno T, Miya A, Kobayashi K, Matsuzuka F, Kakudo K, Kuma K, Miyauchi A. S100A4 expression is an early event of papillary carcinoma of the thyroid. Oncology. 67:397–402. 2004.

4. Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JH, Robertson L, Barraclough R. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 60:1595–1603. 2000.
5. Takenaga K, Nakanishi H, Wada K, Suzuki M, Matsuzaki O, Matsuura A, Endo H. Increased expression of S100A4, a metastasis-associated gene, in human colorectal adenocarcinomas. Clin Cancer Res. 3:2309–2316. 1997.
6. Gupta S, Hussain T, MacLennan GT, Fu P, Patel J, Mukhtar H. Differential expression of S100A2 and S100A4 during progression of human prostate adenocarcinoma. J Clin Oncol. 21:106–112. 2003.

7. Ninomiya I, Ohta T, Fushida S, Endo Y, Hashimoto T, Yagi M, Fujimura T, Nishimura G, Tani T, Shimizu K, Yonemura Y, Heizmann CW, Schfer BW, Sasaki T, Miwa K. Increased expression of S100A4 and its prognostic significance in esophageal squamous cell carcinoma. Int J Oncol. 18:715–720. 2001.

8. Nakamura T, Ajiki T, Murao S, Kamigaki T, Maeda S, Ku Y, Kuroda Y. Prognostic significance of S100A4 expression in gallbladder cancer. Int J Oncol. 20:937–941. 2002.

9. Davies BR, O'Donnell M, Durkan GC, Rudland PS, Barraclough R, Neal DE, Mellon JK. Expression of S100A4 protein is associated with metastasis and reduced survival in human bladder cancer. J Pathol. 196:292–299. 2002.

10. Rosty C, Ueki T, Argani P, Jansen M, Yeo CJ, Cameron JL, Hruban RH, Goggins M. Overexpression of S100A4 in pancreatic ductal adenocarcinoma is associated with poor differentiation and DNA hypomethylation. Am J Pathol. 160:45–50. 2002.
11. Cho YG, Nam SW, Kim TY, Kim YS, Kim CJ, Park JY, Lee JH, Kim HS, Lee JW, Park CH, Song YH, Lee SH, Yoo NJ, Lee JY, Park WS. Overexpression of S100A4 is closely related to the aggressiveness of gastric cancer. APMIS. 111:539–545. 2003.

12. Lee HM, Baek SK, Kwon SY, Jung KY, Chae SW, Hwang SJ, Woo JS, Lee JY. Cyclooxygenase 1 and 2 expressions if the human thyroid gland. Eur Arch Otorhinolaryngol. 263:199–204. 2006.
13. Greene. Frederick L. AJCC cancer staging manual, sixth edition. 67–72. Berlin: Springer-Verlag,. 2006.
14. Andersen PE. Kinsella J, Loree TR. Shaha AR, Shah JP: Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg. 170:467–470. 1995.
16. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 71:414–424. 1990.

17. Zou M, Al-Baradie RS, Al-Hindi H, Farid NR, Shi Y. S100A4 (Mts I) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. British Journal of Cancer. 93:1277–1284. 2005.
18. Takenaga K, Nakanishi H, Wada K, Suzuki M, Matsuzaki O, Matsuura A, Endo H. Increased expression of S100A4, a metastasis-associated gene, in human colorectal adenocarcinomas. Clin Cancer Res. 3:2309–2316. 1997.
19. Komatsu K, Kobune-Fujiwara Y, Andoh A, Ishiguro S, Hunai H, Suzuki N, Kameyama M, Murata K, Miyoshi J, Akedo H, Tatsuta M, Nakamura H. Increased expression of S100A6 at the invading fronts of the primary lesion and liver metastasis in patients with colorectal adenocarcinoma. Br J Cancer. 83(6):769–774. 2000.

20. Kohya N, Kitajima Y, Jiao W, Miyazaki K. Effects of E-cadherin transfection on gene expression of a gallbladder carcinoma cell line: repression of MTS1/S100A4 gene expression. Int J Cancer. 104(1):44–53. 2003.
21. Zou M, Famulski KS, Parhar RS, Baitei E, Al-Mohanna FA, Farid NR, Shi Y. Microarray analysis of metastasis-associated gene expression profiling in a murine model of thyroid carcinoma pulmonary metastasis: identification of S100A4 (Mts1) gene overexpression as a poor prognostic marker for thyroid carcinoma. J Clin Endocrinol Metab. 89:6146–6154. 2004.

22. Lo CY, Lam KY, Leung PP, Luk JM. High prevalence of cyclooxygenase 2 expression in papillary thyroid cacinoma. Eur J Endocrinol. 152:545–550. 2005.
23. Kajita S, Ruebel KH, Casey MB, Nakamura N, Lloyd RV. Role of COX-2, thromboxane A2 synthase, and prostaglandin I2 synthase in papillary thyroid carcinoma growth. Mod Pathol. 18:221–227. 2005.

Fig. 1.
S100A4 and COX2 immunostaining in papillary thyroid carcinoma. It is noted few positive cells in well defined mass (a, S100A4, X200) (c, COX2, X200). There are many strong cytoplasmic or nuclear positive cells in invading fronts (b, S100A4, X200) (d, COX2, X200).
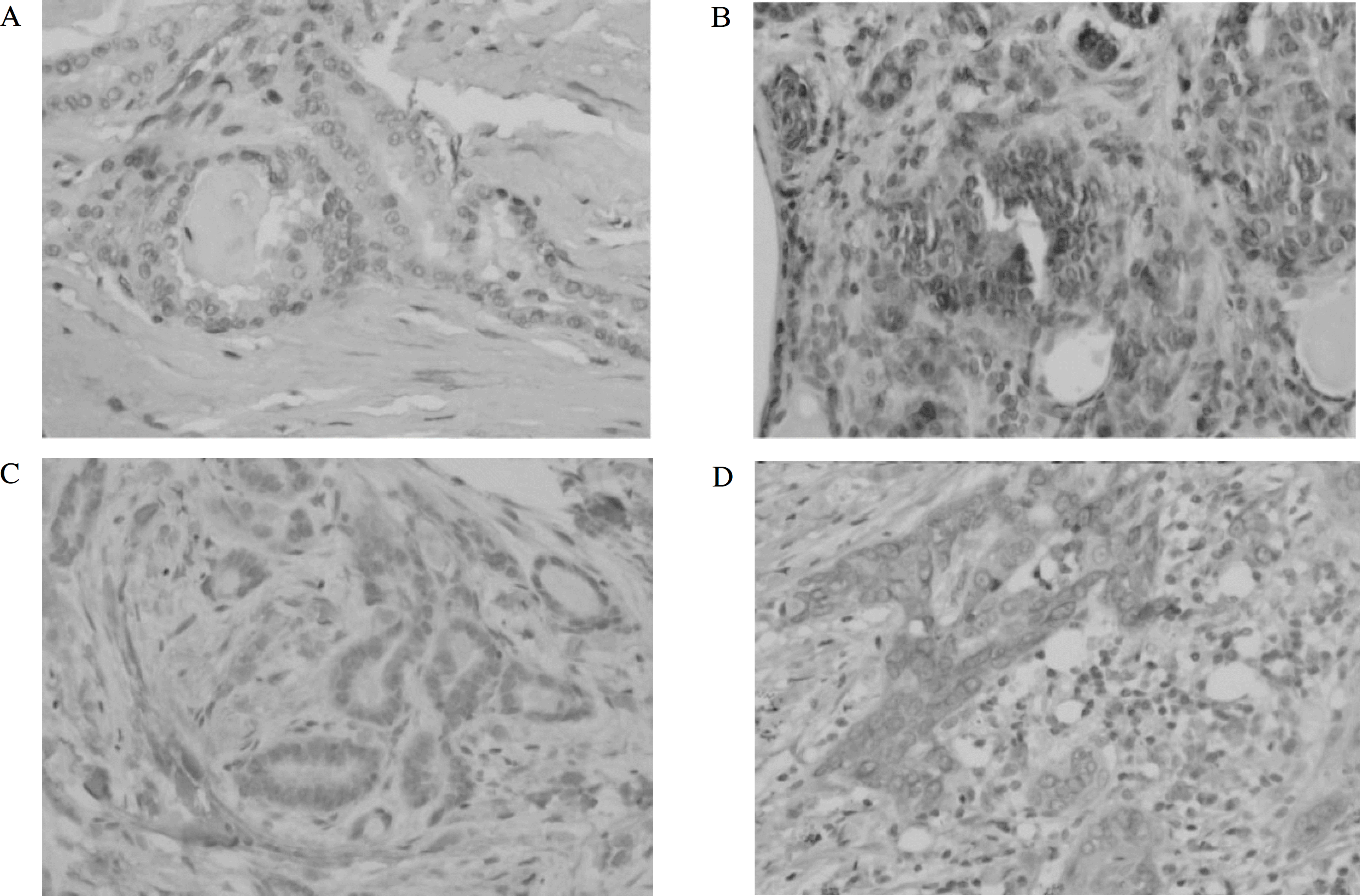
Table 1.
Summary of clinical data and pathological features
| No | Sex | Age | Size | ET ext† | LN meta‡ | Multi§ | pT stage | % reactive COX2 cells II | % reactive S100A4 cells II |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 42 | 1.5 | Y | N | N | pT3 | 35 | 30 |
| 2 | F | 66 | 1.0 | N | N | N | pT1 | 10 | 1 |
| 3 | F | 46 | 0.8 | Y | N | N | pT3 | 30 | 8 |
| 4 | F | 44 | 0.5 | Y | N | N | pT3 | 15 | 40 |
| 5 | F | 29 | 1.0 | Y | Y | N | pT3 | 10 | 40 |
| 6 | F | 46 | 0.6 | Y | Y | Y | pT3 | 14 | 20 |
| 7 | F | 59 | 0.7 | N | N | N | pT1 | 11 | 20 |
| 8 | F | 52 | 0.8 | Y | N | Y | pT3 | 8 | 20 |
| 9 | F | 62 | 1.2 | N | N | N | pT1 | 15 | 15 |
| 10 | F | 71 | 1.7 | Y | N | N | pT3 | 25 | 40 |
| 11 | M | 44 | 0.8 | Y | N | N | pT3 | 24 | 30 |
| 12 | M | 46 | 1.2 | N | N | Y | pT1 | 5 | 18 |
| 13 | M | 29 | 1.5 | N | N | N | pT1 | 12 | 3 |
| 14 | F | 35 | 2.0 | N | N | N | pT2 | 2 | 8 |
| 15 | F | 39 | 3.0 | Y | N | N | pT3 | 20 | 35 |
| 16 | F | 32 | 1.5 | Y | N | N | pT3 | 20 | 15 |
| 17 | F | 60 | 0.8 | Y | N | Y | pT3 | 30 | 12 |
| 18 | F | 40 | 1.5 | Y | N | N | pT3 | 20 | 30 |
| 19 | F | 35 | 0.5 | N | Y | Y | pT1 | 16 | 50 |
| 20 | F | 47 | 1.1 | Y | N | N | pT3 | 21 | 25 |
| 21 | F | 48 | 1.3 | Y | N | N | pT1 | 5 | 4 |
| 22 | F | 54 | 0.7 | Y | N | Y | pT3 | 25 | 17 |
| 23 | M | 38 | 1.5 | N | N | N | pT1 | 20 | 12 |
| 24 | M | 31 | 2.0 | Y | Y | N | pT3 | 30 | 18 |
| 25 | F | 39 | 1.0 | Y | Y | N | pT3 | 14 | 40 |
| 26 | M | 42 | 1.5 | Y | N | Y | pT3 | 21 | 35 |
| 27 | F | 39 | 1.0 | N | N | N | pT1 | 15 | 14 |
| 28 | F | 37 | 0.5 | N | N | N | pT1 | 1 | 1 |
| 29 | F | 36 | 3.0 | Y | Y | N | pT3 | 16 | 8 |
| 30 | F | 54 | 0.4 | Y | N | N | pT3 | 17 | 21 |
| 31 | M | 32 | 2.0 | N | Y | N | pT2 | 10 | 15 |
| 32 | M | 49 | 0.6 | Y | Y | Y | pT3 | 19 | 10 |
| 33 | M | 42 | 0.3 | Y | N | N | pT3 | 15 | 25 |
| 34 | M | 61 | 3.0 | Y | N | Y | pT3 | 22 | 49 |
| 35 | M | 50 | 1.0 | Y | N | N | pT3 | 30 | 4 |
Table 2.
Relationship between S100A4 expression and the clinicopathological parameters
Table 3.
Relationship between COX2 expression and the clinicopathological parameters




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download