Abstract
Radioactive iodine (RAI) therapy is used for the removal of remnant thyroid tissue or metastatic thyroid cancer cells in differentiated thyroid cancer. The main mechanism of the therapy is destruction of cells by radioactive iodine that penetrates the cells though the action of the sodium-iodide symporter (NIS). We experienced a case of a 26-year-old woman with mediastinal uptake as detected on a radioiodine scan, who was previously diagnosed with papillary thyroid cancer. For diagnostic tests including chest computed tomography (CT) and a radioiodine scan, the stimulated thyroglobulin level did not show a definite cause of the mediastinal uptake. During regular follow-up, the thymus became triangular with clear margins. The patient had neither specific symptoms nor physical findings related to the presence of a thymic mass. A subsequent CT scan showed an irregular margin of the thymus, suggestive of thymic metastasis. The patient underwent a mediastinectomy. The removed specimen was composed of normal thymic tissue. Moreover, we demonstrated the presence of human NIS by immunohistochemical analysis. After thymectomy, the mediastinal uptake was markedly decreased as compared to the previous scan. This case suggests that a clinician should be suspicious for the functional uptake of thymus when metastasis is unlikely in a clinical situation.
Figures and Tables
Fig. 1
Radioiodine scans and stimulated thyroglobulin levels. (A) for 2 months after thyroidectomy (post-radioiodine treatment scan). (B) for 14 months after thyroidectomy (post-radioiodine treatment scan). (C) for 36 months after thyroidectomy (post-radioiodine treatment scan). (D) for 8 months after thymectomy (follow-up diagnostic scan): The mediastinal uptake was decreased after thymectomy. TSH, thyroid stimulating hormone; Tg, thyroglobulin; TgAb, antithyroglobulin antibody.
Fig. 2
Chest computed tomography (CT). (A) for postoperative 3 months (B) for postoperative 14 months: The thymus looks like an arrow head and it enlarged than previous CT (A). (C) for postoperative 3 years: The size and shape of thymic lesion was changed. The irregular nodular shape and enhancement in the lesion seems to be a metastatic lesion.
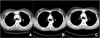
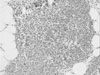
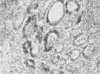
Fig. 3
Immunohistochemistry for hNIS protein in thymic tissue. Only a few thymic macrophages were stained by anti-hNIS (H&E, ×40).
References
1. Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002. 43:1188–1200.
2. Mazzaferri E, Kloos R. Braverman LE, Utiger RD, editors. Carcinoma of follicular epithelium : radioiodine and other treatments. Werner and Ingbar's The Thyroid : A fundamental and clinical text. 2005. 9th ed. Philadelphia: Lippincott Williams & Wilkins;934–966.
3. Vayre L, Sabourin JC, Caillou B, Ducreux M, Schlumberger M, Bidart JM. Immunohistochemical analysis of Na+/I- symporter distribution in human extra-thyroidal tissues. Eur J Endocrinol. 1999. 141:382–386.
4. Meller J, Becker W. The human sodium-iodine symporter (NIS) as a key for specific thymic iodine-131 uptake. Eur J Nucl Med. 2000. 27:473–474.
5. Wilson LM, Barrington SF, Morrison ID, Kettle AG, O'Doherty MJ, Coakley AJ. Therapeutic implications of thymic uptake of radioiodine in thyroid carcinoma. Eur J Nucl Med. 1998. 25:622–628.
6. Davidson J, McDougall IR. How frequently is the thymus seen on whole-body iodine-131 diagnostic and post-treatment scans? Eur J Nucl Med. 2000. 27:425–430.
7. Wapnir IL, van de Rijn M, Nowels K, Amenta PS, Walton K, Montgomery K, Greco RS, Dohan O, Carrasco N. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J Clin Endocrinol Metab. 2003. 88:1880–1888.
8. Park C, Lee M. Thymic radioiodine uptake mimicking metastatic papillary carcinoma in the anterior mediastinum. Korean J Nucl Med. 2002. 36:87–89.
9. Im JG. Im JG, Lee KS, editors. Diseases of mediastinum. Thoracic radiology. 2000. 1st ed. Seoul: Ilchokak;433–459.
10. Nakamura T, Murakami M, Horiguchi H, Nagasaka S, Ishibashi S, Mori M, Ishikawa SE. A case of thymic enlargement in hyperthyroidism in a young woman. Thyroid. 2004. 14:307–310.
11. Salvatori M, Saletnich I, Rufini V, Troncone L. Unusual false-positive radioiodine whole-body scans in patients with differentiated thyroid carcinoma. Clin Nucl Med. 1997. 22:380–384.
12. Tecce PM, Fishman EK, Kuhlman JE. CT evaluation of the anterior mediastinum: spectrum of disease. Radiographics. 1994. 14:973–990.
13. Connolly LP, Connolly SA. T hym ic uptake of radiopharmaceuticals. Clin Nucl Med. 2003. 28:648–651.
14. Spitzweg C, Joba W, Heufelder AE. Expression of thyroid-related genes in human thymus. Thyroid. 1999. 9:133–141.
15. Vermiglio F, Baudin E, Travagli JP, Caillou B, Fragu P, Ricard M, Schlumberger M. Iodine concentration by the thymus in thyroid carcinoma. J Nucl Med. 1996. 37:1830–1831.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download