Abstract
Background
The present study was designed to investigate the correlations of retinoic acid receptor β (RARβ) expression for primary and recurred metastatic lymph node (LN) papillary thyroid carcinoma (PTC) tissues and the correlations of RARβ expression with the uptake of I131 as detected on a whole body scan (WBS).
Methods
Primary and metastatic LN PTC tissues were examined by immunohistochemical methods. Staining positivity was calculated, and staining intensity was graded as negative (0), weak (1+), moderate (2+) and strong (3+). Nuclear staining intensity (NSI) of cells from tissues was also examined.
Results
Seventeen patients who had regional cervical LN metastasis without distant metastasis were included in the study, and 13 patients had the abnormal uptake of I131 as detected on a WBS. In primary PTC tissues, RARβ staining positivity and intensity of carcinoma cells were significantly higher than those of normal cells but NSI was significantly higher in normal cells than carcinoma cells. Between primary and metastatic LN PTC tissues, RARβ staining intensity was correlated after controlling for age. Primary PTC tissues from 14 (82.4%) out of 17 patients were concordant between NSI and the uptake of I131 as detected on a WBS. NSI predicted the I131 uptake as detected on a WBS with 81.3% positive predicted value (PPV) and 100% negative predicted value. Metastatic LN PTC tissues from 13 (76.5%) out of 17 patients were concordant between NSI and the uptake of I131 as detected on a WBS. NSI predicted the uptake of I131 as detected on a WBS with 76.5% PPV. When the results of NSI taken either as positive or negative were correlated with those of the uptake of I131 as detected on a WBS in primary and metastatic LN PTC tissues, the correlation was not significant after controlling for age.
Figures and Tables
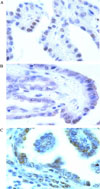 | Fig. 2RARβ immunostaining. A. Normal thyroid cells in primary PTC tissues with positive RARβ staining at nucleus (brown color, ×1000). B. Carcinoma cells in primary PTC tissues with positive RARβ staining in cytoplasm as well as at nucleus (brown color, ×1000). C. Carcinoma cells in metastatic LN PTC tissues with positive RARβ staining (brown color, ×1000). Immunoperoxidase RARβ, hematoxylin counterstaining |
References
1. Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987. 330:444–450.
2. Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990. 345:224–229.
3. Chambon P. A decade of molecular biology of retinoic acid. FASEB J. 1996. 10:940–954.
4. Roberts AB, Sporn MB. Cellular biology and biochemistry of retinoids. The Retinoids. 1984. Orlando: Academic Press;209.
5. Darwiche N, Scita G, Jones C, Rutberg S, Greenwald E, Tennenbaum T, Collins SJ, De Luca LM, Yuspa SH. Loss of retinoic acid receptors in mouse skin and skin tumors is associated with activation of the rasHa oncogene and high risk for premalignant progression. Cancer Res. 1996. 56:4942–4949.
6. Dejean A, Bougeleret L, Grzeschik KH, Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocarcinoma. Nature. 1986. 332:70–72.
7. Gebert JF, Moghal N, Frangioni JV, Sugarbaker DJ, Neel BG. High frequency of retinoic acid receptor abnormalities in human lung cancer. Oncogene. 1991. 6:1859–1868.
8. Hu L, Crowe DL, Rheinwald JG, Chambon P, Gudas U. Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res. 1991. 51:3972–3981.
9. Swisshelm K, Ryan K, Lee X, Tsou HC, Peacocke M, Sager R. Down-regulation of retinoic acid receptor β in mammary carcinoma cell lines and its up-regulation in senescing normal mammary epithelial cells. Cell Growth Differ. 1994. 5:133–141.
10. Geisen C, Denk C, Gremm B, Baust C, Karger A, Bollag W, Schwarz E. High-level of the retinoic acid receptor β gene in normal cells of the uterine cervix is regulated by the retinoic acid receptor α and is abnormally down-regulated in cervical carcinoma cells. Cancer Res. 1997. 57:1460–1467.
11. Caliaro MJ, Marmouget C, Guichard S, Mazars P, Valette A, Moisand A, Bugat R, Jozan S. Response of four human ovarian carcinoma cell lines to all-trans-retinoic acid: relationship with induction of differentiation and retinoic acid receptor expression. Int J Cancer. 1994. 56:743–748.
12. Schreck R, Schnieders F, Schmutzler C, Köhrle J. Retinoids stimulate type I iodothyronine 5'-deiodinase activity in human follicular thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1994. 79:791–798.
13. Rochaix P, Monteil-Onteniente S, Rochette-Egly C, Caratero C, Voigt JJ, Jozan S. Reduced expression of retinoic acid receptor beta protein (RARβ) in human papillary thyroid carcinoma : immunohistochemical and Western blot study. Histopathology. 1998. 33:337–343.
14. Widschwendter M, Berger J, Daxenbichler G, Muller-Holzner E, Widschwendter A, Mayr A, Marth C, Zeimet AG. Loss of retinoic acid receptor β expression in breast cancer and morphologically normal adjacent tissue but not in the normal breast tissue distant from the cancer. Cancer Res. 1997. 57:4158–4161.
15. Houlé B, Rochette-Egly C, Bradley WEC. Tumor-suppressive effect of the retinoic acid receptor β in human epidermoid lung cancer cells. Proc Nat Acad Sci. 1993. 90:985–989.
16. Schmutzler C. Regulation of the sodium/iodide symporter by retinoids-a review. Exp Clin Endocrinol Diabetes. 2001. 109:41–44.
17. Dohan O, Baloch Z, Banrevi Z, Livolsi V, Carrasco N. Predominant intracellular overexpression of the Na+/I- symporter (NIS) in a large sampling of thyroid cancer cases. J Clin Endocrinol Metab. 2001. 86:2697–2700.
18. Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989. 63:908–911.
19. American Joint Committee on Cancer. Cancer staging manual. 2002. New York: Springer-Verlag;77–87.
20. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993. 114:1050–1058.
21. Del Senno L, Rossi R, Gandini D, Piva R, Franceschetti P, degli Uberti EC. Retinoic acid-induced decrease of DNA synthesis and peroxidase mRNA levels in human thyroid cells expressing retinoic acid receptor α mRNA. Life Sci. 1993. 53:1039–1048.
22. Bassi V, Vitale M, Feliciello A, De Riu S, Rossi G, Fenzi G. Retinoic acid induces intercellular adhesion molecule-1 hyperexpression in human thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1995. 80:1129–1135.
23. Kurebayashi J, Tanaka K, Otsuki T, Moriya T, Kunisue H, Uno M, Sonoo H. All-trans-retinoic acid modulates expression levels of thyroglobulin and cytokines in a new human poorly differentiated papillary thyroid carcinoma cell line, KTC-1. J Clin Endocrinol Metab. 2000. 85:2889–2896.
24. Schmutzler C, Brtko J, Winzer R, Jakobs TC, Meissner-Weigl J, Simon D, Goretzki PE, Kohrle J. Functional retinoid and thyroid hormone receptors in human thyroid-carcinoma cell lines and tissues. Int J Cancer. 1998. 76:368–376.
25. Schmutzler C, Brtko J, Bienert K, Kohrle J. Effects of retinoids and role of retinoic acid receptors in human thyroid carcinomas and cell lines derived therefrom. Exp Clin Endocrinol Diabetes. 1996. 104:Suppl 4. 16–19.
26. Haugen BR, Larson LL, Pugazhenthi U, Hays WR, Klopper JP, Kramer CA, Sharma V. Retinoic acid and retinoid X receptors are differentially expressed in thyroid cancer and thyroid carcinoma cell lines and predict response to treatment with retinoids. J Clin Endocrinol Metab. 2004. 89:272–280.
27. Hoque MO, Rosenbaum E, Westra WH, Xing M, Ladenson P, Zeiger MA, Sidransky D, Umbricht CB. Quantitative assessment of promoter methylation profiles in thyroid neoplasms. J Clin Endocrinol Metab. 2005. 90:4011–4018.
28. Carrasco N. Iodide transport in the thyroid gland. Biochem Biophys Acta. 1993. 1154:65–82.
29. Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996. 379:458–460.
30. Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, Jhiang SM. Cloning of the human sodium symporter. Biochem Biophys Res Commun. 1996. 226:339–345.
31. Eskandari S, Loo DDF, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I-. mechanism, stoichiometry, and specificity. J Biol Chem. 1997. 272:27230–27238.
32. Schmutzler C, Kohrle J. Implication of the molecular characterization of the sodium-iodide symporter (NIS). Exp Clin Endocrinol Diabetes. 1998. 106:S1–S10.
33. Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M. Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 1999. 141:443–457.
34. Arturi F, Russo D, Giuffrida D, Schlumberger M, Filetti S. Sodium-iodide symporter (NIS) gene expression in lymph-node metastases of papillary thyroid carcinomas. Eur J Endocrinol. 2000. 143:623–627.
35. Schmutzler C, Winzer R, Meissner-Weigl J, Köhrle J. Retinoic acid increases sodium/iodide symporter mRNA levels in human thyroid cancer cell lines and suppresses expression of functional symporter in nontransformed FRTL-5 rat thyroid cells. Biochem Biophys Res Commun. 1997. 240:832–838.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download