Abstract
Background
Graves' disease (GD) is an organ-specific autoimmune disease that is characterized by lymphocyte infiltration of the thyroid, which finally leads to follicular destruction. The CD4+CD25+ regulatory T cells are important for maintaining peripheral tolerance to self-antigens and impaired activity can cause autoimmune diseases. CD137 (4-1BB), a member of the tumor necrosis factor receptor superfamily and expressed on activated T cells, is a candidate molecule for a co-stimulatory role in autoimmune thyroid disease. In this study, we aimed to assay the frequency of CD4+CD25+ T cells in GD patients and to investigate the role of CD137-mediated costimulation in CD4+CD25+ T cells.
Methods
The frequencies of the CD4+CD25+ T cells in the peripheral blood (PB) of GD patients were determined by flow cytometric analysis. After the CD4+CD25+ T cells were isolated from PB mononuclear cells (PBMC) of the GD patients using immunomagnetic beads, the functional activity of the CD4+CD25+ T cells was characterized by use of a proliferation assay. mRNA expression of Foxp 3 in the CD4+CD25+ T cells of the GD patients was observed by real-time RT-PCR.
Results
In this study, we found that GD patients had a low proportion of CD4+CD25+ T cells (mean ± SD; 1.47 ± 0.31%) in PBMC as compared with normal subjects. CD137-mediated costimulation increased the expression of CD25 and Foxp 3 in CD4+ T cells in GD patients as compared with normal subjects. Moreover, the CD137-mediated costimulation also induced the proliferation of CD4+CD25+ T cells in GD patients, and the expanded CD4+CD25+ T cells could suppress other CD4+CD25- T cells in a co-culture.
Figures and Tables
 | Fig. 1The frequency of CD137+ cells in CD4+CD25+ T cells of the patients with Grave's disease. The frequency of CD25+CD4+ T cells in PBMC of GD patients and normal subjects were analyzed by flow cytometry with PE-labelled anti-CD137 staining. The values are expressed as mean ± SD of triplicate data. P < 0.0001 vs. normal group. |
 | Fig. 2The CD4+CD25+ T cell population in PBMC of patients with Graves' disease. PBMC were stimulated by plate-bound OKT3 (1 µg/mL), soluble anti-CD28 (1 µg/mL) and/or anti-CD137 (1 µg/mL) for 24 h. The cells in these analyses were gated on lymphocytes via forward and side scatter properties in flow cytometry. The values are expressed as mean ± SD of triplicate data. P < 0.05, P < 0.001 and P < 0.0001 vs. normal group. |
 | Fig. 3Proliferation of CD4+CD25+ T cells by anti-CD137 stimulation. The CD4+CD25+ T cells (5 × 104 cells) were stimulated with APC (2 × 104 cells/mL), plate-bound anti-CD3 (1 µg/mL), soluble anti-CD28 (1 µg/mL) and IL-2 (20 U/mL) at presence or absence with anti-CD137 mAb (4B4; 1 µg/mL). Proliferation was determined at day 3, with [3H]thymidine added for the last 18h of culture. The values are expressed as mean ± SD of triplicate data (SD = bar). P < 0.05 and P < 0.001 vs. normal group. |
 | Fig. 4The ability of the CD4+CD25+ T cells to suppress the proliferation of the cocultured CD4+CD25- T cells. CD4+CD25+ T cells (2 × 104 cells) were stimulated alone and in coculture with CD4+CD25- T cells (2 × 104 cells) in the presence of APC (2 × 104 cells). The CD4+CD25- T cells were also stimulated alone. Cells were stimulated with plate-bound anti-CD3(1 µg/mL) and soluble anti-CD28 (1 µg/mL) at presence or absence with anti-CD137 mAb (4B4; 1 µg/mL). Proliferation was determined at day 3, with [3H]thymidine added for the last 18h of culture. |
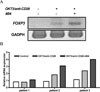 | Fig. 5Expression of Foxp 3 in CD25+CD4+ T cells of GD patients. The CD4+CD25+ T cells were stimulated with OKT3/anti-CD28 (1 µg/mL) alone or with 4B4(1 µg/mL). After mRNA was isolated from cells using TRIzol reagent, cDNA was reverse transcribed and then amplified by PCR. Expression of Foxp 3 gene in CD4+CD25+ T cells of GD patients was determined by reverse transciption-PCR (A) and real time PCR (B). |
References
1. Mathis D, Benoist C. Back to central tolerance. Immunity. 2004. 20:509–516.
2. Heufelder AE. Pathogenesis of ophthalmopathy in autoimmune thyroid disease. Rev Endocr Metab Disord. 2000. 1:87–95.
3. Weetman AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol. 2003. 148:1–9.
4. Salmaso C, Olive D, Pesce G, Bagnasco M. Costimulatory molecules and autoimmune thyroid diseases. Autoimmunity. 2002. 35:159–167.
5. Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003. 14:265–273.
6. Kim ES, Jung HW, Nam-Goong IS, Woo SJ, Choi JI, Kim YI. Expression of 4-1BB and 4-1BBL in Graves' disease. J Kor Soc Endocrinol. 2006. 21:116–124.
7. Mittler RS, Foell J, McCausland M, Strahotin S, Niu L, Bapat L, Hewes LB. Anti-CD137 antibodies in the treatment of autoimmune disease and cancer. Immunol Res. 2004. 29:197–208.
8. Foell J, Strahotin S, O'Neil SP, McCausland MM, Suwyn C, Haber M, Chander PN, Bapat AS, Yan XJ, Chiorazzi M, Hoffmann MK, Mittler RS. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest. 2003. 111:1505–1518.
9. Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu XY. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002. 168:1457. 1465.
10. Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 4 1BB mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004. 10:1088–1094.
11. Roncarolo M, Levings MK. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol. 2000. 12:676–683.
12. Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000. 18:423–449.
13. Zwar TD, VAN Driel IR, Gleeson PA. Guarding the immune system: Suppression of autoimmunity by CD4CD25 immunoregulatory T cells. Immunol Cell Biol. 2006. 84:487–501.
14. Morris GP, Kong YC. Interference with CD4+CD25+ T-cell-mediated tolerance to experimental autoimmune thyroiditis by glucocorticoid-induced tumor necrosis factor receptor monoclonal antibody. J Autoimmun. 2006. 26:24–31.
15. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.
16. Fontenot JD, Gavin MA, Rudensky AY. Foxp 3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003. 4:330–336.
17. Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutteing edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp 3 induction and down-regulation of Smad7. J Immunol. 2004. 172:5149–5153.
18. Saitoh O, Nagayama Y. Regulation of Graves' hyperthyroidism with naturally occurring CD4+CD25+ regulatory T cells in a mouse model. Endocrinology. 2006. 147:2417–2422.
19. Ludgate M, Baker G. Inducing Graves' ophthalmopathy. J Endocrinol Invest. 2004. 27:211–215.
20. Furugaki K, Shirasawa S, Ishikawa N, Ito K, Ito K, Kubota S, Kuma K, Tamai H, Akamizu T, Hiratani H, Tanaka M, Sasazuki T. Association of the T-cell regulatory gene CTLA4 with Graves' disease and autoimmune thyroid disease in the Japanese. J Hum Genet. 2004. 49:166–168.
21. Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-b and CTLA-4. J Immunol. 2003. 171:5012–5017.
22. Morris GP, Kong YC. Interference with CD4+CD25- T-cell-mediated tolerance to experimental autoimmune thyroiditis by glucocorticoid-induced tumor necrosis factor receptor monoclonal antibody. J Autoimmun. 2006. 26:24–31.
23. Bettelli E, Dastrange M, Oukka M. Foxp 3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci. 2005. 102:5138–5143.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download