Abstract
Background
Papillary thyroid carcinoma is among the most curable cancers, but some patients are at high risk for recurrence or even death. MHC antigens are essential molecules for the pathogenesis of carcinoma and also the physiologic immune responses against tumor. However, there is no data about the relationship between the expression of MHC antigens and the clinical prognosis of papillary thyroid carcinoma patients.
Methods
We analyzed the relationship between the various prognostic factors and the MHC antigen expression by conducting a retrospective study of 215 patients, who had undergone thyroidectomy for papillary thyroid carcinoma between 1987 and 2003.
Results
The expressions of MHC class II antigens were more frequent in papillary thyroid carcinoma than in the other thyroid diseases. Yet there was no statistically significant relationship between most of the clinicopathological factors and the expression of MHC class II antigens in papillary thyroid carcinoma patients. Interestingly, an HLA-DR expression was found in 8 (30.8%) of the 26 patients in the recurrence group and in 13 (76.5%) of the 17 patients in the non-recurrence group, and HLA-DP/DQ immunoreactivity was positive in 10 (38.5%) cases of the recurrence group and in 14 (82.4%) cases of the non-recurrence group.
Conclusion
Papillary thyroid carcinoma showed a more frequent expression of MHC Class II antigens. However, the recurred papillary thyroid carcinoma showed a tendency to downregulate the expression of MHC class II antigens. Hence, the molecular mechanism for the expression of MHC class II antigens might have a role in the recurrence of papillary thyroid carcinoma.
Figures and Tables
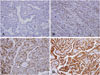 | Fig. 1Immunohistochemical staining of MHC class II antigen expression in papillary thyroid carcinoma.
(A, B) Immunohistochemistry in thyroid epithelium of papillary thyroid carcinoma showed negative value (grade 0 and 1). Original magnification: × 200. (C, D) Immunohistochemistry detecting the MHC class II antigen in papillary thyroid carcinoma showed positive value (grade 2 and 3). Original magnification: × 200.
|
Table 1
Comparisons of MHC class II antigen expressions between malignant diseases, Hashimoto's thyroiditis and other benign diseases

*Malignant disease; papillary thyroid carcinoma (138), follicular thyroid carcinoma (28), medullary thyroid carcinoma (4), anaplastic thyroid carcinoma (3).
†Other benign disease; nodular hyperplasia (6), diffuse hyperplasia (5), subacute thyroiditis (2), follicular adenoma (21).
‡Malignant disease vs. Other benign disease, Pearson Chi-Square test.
Table 2
Comparisons of MHC class II antigen expression between follicular thyroid carcinoma and papillary thyroid carcinoma

Table 3
Comparisons between clinical and pathological factors according to the MHC class II antigen expression

References
1. Korea Central Cancer Registry, Ministry of Health and Welfare Republic of Korea. 2002 annual report of the Korea Central Cancer Registry (based on registered data from 139 hospitals). 2003.
2. Hundal SA, Fleming ID, Fremgen AM, Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998. 83:2638–2648.
3. Franceschi S, Boyle P, Maisonneuve P, La Vecchia C, Burt AD, Kerr DJ, MacFarlane GJ. The epidemiology of thyroid carcinoma. Crit Rev Oncog. 1993. 4:25–52.
4. Kang JM, Kim TS, Noh DY, Youn YK, Choe KJ, Oh SK. Prognostic factors for locally invasive papillary thyroid carcinomas. J Korea Surg Soc. 2000. 59:478–487.
5. Schlumberger M, Tubiana M, De Vathaire F, Hill C, Gardet P, Travagli JP, Fragu P, Lumbroso J, Caillou B, Parmentier C. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986. 63:960–967.
6. Jager D, Jager E, Knuth A. Immune responses to tumor antigens: implications for antigen specific immunotherapy of cancer. J Clin Pathol. 2001. 54:669–674.
7. Matoba K, Iizuka N, Gondo T, Ishihara T, Yamada-Okabe H, Tamesa T, Takemoto N, Hashimoto K, Sakamoto K, Miyamoto T, Uchimura S, Hamamoto Y, Oka M. Tumor HLA-DR expression linked to early intrahepatic recurrence of hepatocellular carcinoma. Int J Cancer. 2005. 115:231–240.
8. Booman M, Douwes J, Gals AM, Reimersma SA, Jordanova ES, Kok K, Rosenwald A, de Jong D, Schuuring E, Kluin PM. Mechanisms and effects of loss of human leukocyte antigen class II expression in immune-privileged site-associated B-cell lymphoma. Clin Cancer Res. 2006. 12:2698–2705.
9. Hwang ES, Kim DW, Hwang JH, Jung HS, Suh JM, Park YJ, Chung HK, Song JH, Park KC, Park SH, Yun HJ, Kim JM, Shong M. Regulation of signal transducer and activator of transcription 1 (STAT1) and STAT1-dependent genes by RET/PTC (rearranged in transformation/papillary thyroid carcinoma) oncogenic tyrosine kinases. Mol Endocrinol. 2004. 18:2672–2684.
10. Cooper SC, Doherty GH, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, Mclver B, Sherman SI, Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006. 16:109–142.
11. Kim KS, Lyu JS, Hong SJ, Kim WB, Song YK. Serum thyroglobulin levels predicting recurrence and distant metastasis after surgery in patients with differentiated thyroid cancer. J Korea Soc Endocrinol. 2003. 18:153–165.
12. Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001. 86:1447–1463.
13. Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001. 411:380–384.
14. Engelhard VH. Structure of peptides associated with class I and class II MHC molecules. Annu Rev Immunol. 1994. 12:181–207.
15. Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol. 2005. 42:501–510.
16. Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004. 53:904–910.
17. Redondo M, Garcia J, Villar E, Rodrigo I, Perea-Milla E, Serrano A, Morell M. Major histocompatibility complex status in breast carcinogenesis and relationship to apoptosis. Hum Pathol. 2003. 34:1283–1289.
18. Warabi M, Kitagawa M, Hirokawa K. Loss of MHC class II expression is associated with a decrease of tumor-infiltrating T cells and an increase of metastatic potential of colorectal cancer: immunohistological and histopathological analyses as compared with normal colonic mucosa and adenoma. Pathol Res Pract. 2000. 196:807–815.
19. Cho KJ, Jang JJ. Expression of ras oncogene product, MHC class II antigens and human papillomavirus 16/18DNA in carcinomas of the uterine cervix. J Korea Soc Pathol. 1993. 27:485–490.
20. Maudsley DJ, Pound JD. Modulation of MHC antigen expression by viruses and oncogenes. Immunol Today. 1991. 12:429–431.
21. Morris A. Modification of histocompatibility antigen expression in cells expressing activated oncogene: Implications for tumor development. Anticancer Res. 1990. 10:1161–1168.
22. Hayashi H, Tanaka K, Jay F, Khoury G, Jay G. Modulation of the tumorigenicity of human adenovirus-12-transformed cells by interferon. Cell. 1985. 43:263–267.
23. Gopas J, Rager-Zisman B, Bar-Eli M, Hammumerling GJ, Segal S. The relationship between MHC antigen expression and metastasis. Adv Cancer Res. 1989. 53:89–115.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download