Abstract
Purpose
In this study the reliability and validity of the Korean version of the Cancer Stigma Scale (KCSS) was evaluated.
Methods
The KCSS was formed through translation and modification of Cataldo Lung Cancer Stigma Scale. The KCSS, Psychological Symptom Inventory (PSI), and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire - Core 30 (EORTC QLQ-C30) were administered to 247 men and women diagnosed with one of the five major cancers. Construct validity, item convergent and discriminant validity, concurrent validity, known-group validity, and internal consistency reliability of the KCSS were evaluated.
Results
Exploratory factor analysis supported the construct validity with a six-factor solution; that explained 65.7% of the total variance. The six-factor model was validated by confirmatory factor analysis (Q (χ2/df)= 2.28, GFI=.84, AGFI=.81, NFI=.80, TLI=.86, RMR=.03, and RMSEA=.07). Concurrent validity was demonstrated with the QLQ-C30 (global: r=-.44; functional: r=-.19; symptom: r=.42). The KCSS had known-group validity. Cronbach's alpha coefficient for the 24 items was .89.
Cancer is the leading cause of death in men and women worldwide. Cancer incidence was at 224,177 with the five-year survival rate reported 69.4% in 2013 in Korea [1], while in the United States, it was 69.0% in 2011 [2]. With cancer patient survival rates increasing, national policy and clinical researchers have begun to focus on the management of psychological distress and quality of life of cancer patients [3].
More than 30.0% of cancer survivors have a negative attitude toward cancer, and the cancer patients who experienced a stigma toward cancer showed more than 2.5 times higher rates of depression compared to cancer patients with positive attitude toward cancer without a stigma [4]. Stigma was linked to guilt and shame, self-blame, blame attribution, and depression, and in cancer research, these concepts were associated with one another [56]. Stigma may have properties contrary to social identity and, therefore, those with cancer may feel undervalued or tagged, and may experience negative stereotyping and discrimination, which together lead to social rejection, social isolation, lack of social support, and low social status [7]. In particular, health-related stigma (HRS) represents experiencing rejection, blame, or devaluation due to his or her illness [89]. HRS has been associated with illness-induced stress and contributing to psychological, physical, and social morbidity [910]; such that a negative psychosocial impact results in depression, receiving limited social support, decreased treatment adherence, and adverse treatment effects [11]. Because HRS can be an obstacle to seeking professional help and health promotion activities [8], it is important for both clinical research and practice professionals to use a valid and reliable tool to periodically evaluate cancer patients for HRS and effectively manage HRS.
As cancer can be a fatal disease, it is very important to find interventions to improve psychosocial and physical health for cancer survivors to help them avoid long-term ill-effects and possible death. There are increasing empirical studies on how stigma affects cancer survivors [12]. Although researchers found the fact that cancer stigma affects individuals with cancer, most of the studies focus on patients with lung cancer [8111314]. Van Brakel [9] reported that many instruments have been developed to assess the intensity and qualities of stigma, but these are often condition-specific including cancer type-specific. Stigma has an effect on individuals and their families, as well as on the effectiveness of health programs. The similarity in the consequences of stigma in many different cancer's types [12] suggests that the development of generic instruments to assess HRS of all types of cancer patients may be possible [9].
Through a literature review of 63 research papers that addressed the issues related to measuring stigma or stigma-related constructs, and that contained a sample of the instrument or items used, aspects of HRS can be grouped into five categories. First, the experience of actual discrimination and/or participation restrictions on the part of the person affected; second, attitudes towards the people affected; third, perceived or felt stigma; fourth, self or internalized stigma; and fifth, discriminatory and stigmatizing practices in health services, legislation, media and educational materials [9]. While Cataldo Lung Cancer Stigma Scale (CLCSS) developed by Cataldo et al. [13] is a multidimensional measurement tool that can measure all aspects of HRS, it was developed to measure the perceived stigma of lung cancer patients. It consists of four subscales, stigma and shame domain, social isolation domain, discrimination domain, and smoking status domain and it has been reported to have a satisfactory level of validity and high reliability at the time of development. In a study of cancer-related stigma by Else-Quest et al. [12], it was reported that cancer patients including patients with lung cancer have internal causal attributions where they believe cancer occurred due to their harmful lifestyle habits. So, most cancers can conjure a similar attribution of blame as is found with lung cancer because most cancers are frequently associated with individual's behaviors. Because of the lack of a valid and reliable tool, empirical evidence of cancer-related stigma is limited. Thus, to develop a generic cancer stigma scale, the existing instruments should be further validated, developed, or adapted for generic use, where possible.
In particular, the measurement tools for cancer stigma were as follow: (a) a single item for perceived stigma, "People judge me for my type of cancer," was rated 1 (strongly disagree) to 5 (strongly agree) [12]; and (b) CLCSS [13]. The CLCSS has been used in Korea and China [815]. The CLCSS was adapted to 123 lung cancer patients without testing the validity in the Korean patient population [8]. Therefore, we modified the CLCSS to form the Korean Cancer Stigma Scale (KCSS) and conducted a study to test the validity of the KCSS in patients with one of the five major types of cancer in Korea (breast, colon, lung, stomach, and uterine cervix cancer). This paper is to report the validity and reliability of the KCSS.
The study was a cross-sectional survey design using a convenience sample and self-administered questionnaire.
The convenience sample used in this study was comprised of 247 inpatients and outpatients (18 years or older) diagnosed with breast, colon, lung, stomach, or uterine cervix cancer. As of 2013, when it comes to cancer incidence in Korean men and women, frequency is in the order of gastric, colon, lung, liver, prostate, and thyroid gland in men and thyroid gland, breast, colon, gastric, lung, liver, uterine cervix in women [1]. When it comes to cancer locations to select for the research participants, breast cancer which is the second place in women and uterine cervix cancer which is the seventh place were selected to have the same ratio of gender, together with gastric cancer, colon cancer, and lung cancer patients which are common cancers for both men and women. Thyroid cancer was excluded from this study because the prognosis is excellent compared to other types of cancer while it ranks in the sixth place in men and first place in women.
This study was conducted at C National University H Hospital cancer center in C Province, Korea. Three trained interviewers collected data. In order to conduct the exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) for the construct validity verification, the numbers of participants that are required is at least 200 or 4 times of number of items [16]. The sample size in this study was 247 for 31 items, thus, satisfied the requirements for sample size.
The CLCSS, developed in 2011 [13], includes 31 items and 4 subscales: stigma and shame, social isolation, discrimination, and smoking status. Each stigma item was measured using a four-point Likert-type scale ranging from 1 (strongly disagree) to 4 (strongly agree) with the higher scores indicating a stronger stigma perceived by the patient. The possible scores ranged from 31~124. Coefficient alphas ranged from .75 to .97 for the subscales (.97 for stigma and shame, .96 for social isolation, .92 for discrimination, and .75 for smoking status) and .96 for the overall CLCSS.
The KCSS was formed using the cultural adaptation processes suggested by the World Health Organization [17]. First, after obtaining permission to translate the CLCSS and modify the items so that they could be applied to the five major types of cancer patients in Korea, two bilingual nursing professors translated the CLCSS from English to Korean and produced a preliminary draft. The draft underwent a process of convergence through mutual discussion regarding differences. The translated draft was then back-translated to English by an English expert. A comparison was made between the original and back-translated CLCSS which yielded no substantial differences.
Second, the term "lung cancer" was changed to "cancer" for 31 items. Item No. 10, "Smokers could be refused treatment for lung cancer" was changed to "Cancer patients who have high risk factors (i.e., smoking, obese, salty food intake, genetic, etc.) could be refused cancer treatment" and the item No. 30, "Healthcare providers don't take 'smoker's cough' seriously" was changed to "Healthcare providers don't take 'cancer patients' signs' seriously (i.e., coughing for lung cancer, constipation for colon cancer, lumps for breast cancer, abnormal bleeding for cervix cancer, and gastric fullness and indigestion for stomach cancer)." Also, changes were made in item No. 28, "stop smoking" and item No. 29, "smoking cessation" by combining No 28 with Item No. 29 as, "Despite not usually having harmful lifestyle habits, people think it was my fault that I had a cancer." Therefore, in the total of 31 items of the original CLCSS, items 28 and 29 were revised to 29, and the interim version of the KCSS had a final of 30 items.
Third, for verification of the content validity for the interim version of the KCSS which went through the process of translation and back-translation, we selected the professional group of three nursing professors and one oncology nurse as suggested by Lynn [18]; that is the desirable number of professionals for content validity verification should be more than 3 but less than 10. The validity of each item was designed to be assessed from score 4 of 'Strongly agree' to score 1 of 'Strongly disagree', then calculation was done for the content validity index (CVI) of each item. As a result, all of CVI of 30 items were above 80.0%.
Fourth, to test feasibility, fifteen patients with five types of cancer were tested using the scale as a pilot test. Further revisions of vocabulary and clarity were made based on the feedback of the 15 patients to generate the final scale; this took about 10 minutes for the questionnaire responses. Patients responded to each item using a 4-point Likert scale with the higher scores representing stronger stigma perceived by the patient. The total score of the KCSS ranged from 30 to 120.
To evaluate the concurrent validity of the KCSS, we used the Korean version of the quality of life questionnaire (QLQ-C30) version 3.0 [19]; permission to use the instrument was obtained via electronic communication. According to previous studies, the quality of life of cancer patients is closely linked to cancer stigma; that is, the worse the quality of life, the greater the cancer stigma the patient presents [81214]. The QLQ-C30 seems to be an adequate instrument to verify the concurrent validity of the KCSS because the degree of perceived cancer stigma suffered by patients with cancer can affect their perceptions of cancer-related quality of life. This instrument included three subscales: global health status (2 items), functional (15 items assessing physical, role, emotional, cognitive, and social aspects), and symptom (13 items assessing fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial problems). The two global health status-related items were scored using a 7-point scale, and the functional and symptom-related items were scored using a 4-point scale. The scores were converted to a 100-point scale according to the scoring manual [20] and the scores ranged from 0~100, with QOL increasing in proportion to the global health status and functional subscale scores; in contrast, the lower the symptom subscale score, the higher the QOL. In a study by Yun and colleagues [19], the Cronbach's alpha coefficient was .70 or higher in all but the cognitive functional scale (.60). In this study, the Cronbach's alphas were .80, .87, and .84 for the global health status, functional, and symptom subscales, respectively.
To evaluate the known-group validity of the KCSS, we used Korean version of the psychological symptom inventory (PSI) developed by the National Cancer Center [3] which measure psychological distress that was proposed as closely related variables to the cancer stigma in previous studies [4581114], and use of the instrument was confirmed through an e-mail. The PSI assessed the current distress levels of three symptoms and interference with daily activities (insomnia, anxiety, and depression).
Insomnia, anxiety, and depression domain consists of one question of symptom-related severity and one question of interference with daily activity respectively and each item configured by domain was measured using a bipolar anchor scale ranging from 0 (absolutely not or no disrupting daily life) to 10 (extremely severe or completely disrupting daily life). It means that the score range for each domain is from 0 to 20 and the higher the score, the higher insomnia, anxiety, and depression. When the cut-off score for each domain is 4 or more in both severity and interference, it means that there is psychological distress. In the present study, Cronbach's alpha was .83 in insomnia and interference with daily activity, .84 in anxiety and its interference, and .88 in depression and its interference.
The study procedures were approved by the institutional review board (IRB) of C National University H Hospital, located in C Province (IRB No 2012-167). After reading the informed consent form and giving written consent, submission of the completed questionnaires implied that participants consented to participate in this study. Ethical consideration information about the research was given to these participants.
Descriptive statistics and appropriate reliability and validity statistical tests were used with SPSS version 21.0 and Amos 21.0. Descriptive statistics were used to establish the frequency, range, mean, and standard deviation of the demographic and clinical characteristics of the main sample.
For reliability assessment, Cronbach's alpha coefficients were conducted. For the validity assessment, construct validity, item convergent and discriminant validity, concurrent validity, and known-group validity were evaluated. For construct validity, EFA and CFA were performed. First, we conducted KMO (Kaiser-Meyer-Olkin) and Bartlett Sphericity test in order to confirm whether the materials that were collected prior to the factor analysis are appropriated for factor analysis. We used the eigenvalue of one and above for the factor extraction by the EFA, cumulative percentage 60.0% and above for variance that is explained by the extracted factors, and .50 and above for factor loading criterion [21]. The model verification of CFA can be conducted on the basis of the Q (χ2/df), GFI (goodness of fit index), AGFI (adjusted goodness of fit index), NFI (normed fit index), TLI (Tucker-Lewis coefficient), RMR (root mean square residual), and RMSEA (root mean square error of approximation). It is acceptable when Q value is 3.0 or less and the model fit is judged to be good if GFI, AGFI, NFI, and TLI are .90 or greater and RMR is .05 or less [22]. And RMSEA is a good model if it is below .05 and if it is .05 to .08, it is considered to be a suitable model [21].
To test convergent and discriminant validity of KCSS items, multi-trait multi-item matrix analysis was implemented. In multi-trait multi-item matrix analysis, item convergent validity was judged satisfied if item–subscale correlation corrected for overlap for coefficients should be .40 or greater [23], and also in item discriminant validity, discriminant validity was judged satisfactory if the difference between the item-own and item-other subscale correlation is greater than 2 times the standard error of correlation coefficients, it can be considered that the item discriminant validity is established [2324].
Concurrent validity was determined by calculating Pearson correlation coefficients for relationships between the 24-item KCSS and subscales of QLQ-C30. To assess the known-group validity of the instrument, the differences in KCSS scores according to the distress group classification were analyzed with independent t-test.
All tests used were two-sided, and a p value of less than 5% was considered statistically significant. In total, 247 data were analyzed.
The study sample comprised 247 patients; 20.6% had breast cancer; 20.2%, colon cancer; 20.2%, uterine cervical cancer; 20.0%, gastric cancer; and 19.0%, lung cancer. The average age was 57.26 (SD=12.25 year, range 24~85 years old), 60.3% were women, 87.0% were married, 65.6% had a religion, and 78.9% had a job. Educational level was 36.8% with high school graduation and 23.5% with middle school graduation. At the time of the survey, 58 patients (23.5%) were diagnosed with stage I disease, 41 (16.6%) had stage II disease, 47 (19.0%) had stage III disease, and 101 (40.9%) had stage IV disease. Types of current treatment reported were anticancer chemotherapy (68.5%) and operation and radiation therapy (14.0% or more each).
Responses for each psychometric variable (i.e., KCSS, QLQ-C30, and PSI) were positively skewed. Participants responded with a positive tendency for stigma. The floor effect in each subscale of KCSS ranged from 4.2% to 14.8%, and the ceiling effect ranged from 0.4% to 0.8%, and had less than 15.0% which is acceptable criteria in all subscales [25]. Also the skewness of each KCSS item ranged from -0.52 to 2.62 and kurtosis ranged from -0.36 to 5.08. In this study, as skewness was not greater than the absolute value 3 and kurtosis was not greater than the absolute value 10, it has been confirmed that it does not deviate from the univariate normal distribution [26].
The total stigma scale (KCSS) score was 35.28±9.12. Quality of life (QLQ-C30) was moderate; the global QOL score was 54.86±20.26, and the symptom QOL and functional QOL were 28.69±16.34 and 71.02±16.42, respectively. The score of distress (PSI) was average of 5.04±2.61 for insomnia, 4.92±2.35 for anxiety, and 4.54±2.53 for depression.
To test for the adequacy of the sample size, the authors examined the correlation matrix using the KMO [27]; the values of KMO of the first 30 items and last 24 items were .84 and .86 (p<.001), which were all appropriate. The Bartlett Sphericity test is used to evaluate whether the correlation matrix is fit for the factor analysis [21]; the chi-squared was 2200.94 (degree of freedom=276), which was statistically significant (p<.001), indicating that factor analysis of KCSS was appropriate.
We used the principal component factor analysis as factor extract model that is mainly used to minimize the information loss with the minimum factor aiming the forecast and used varimax rotation that classifies the factors by maximizing the sum of factor loading variance and clear the factor property at the most [21]. The initial run of the EFA using an eigenvalue curve indicates eight eigenvalues in the scree plot above the mean eigenvalue, so these eight factors were retained. After varimax rotation, one item (No. 7) with a factor loading of <.40 was deleted and items with a factor loading of <.50 were deleted (Items No. 2, 10, 20, 24, 26). Rotation was again performed and six factors were extracted; these factors could explain 65.7% of the total variance. KCSS showed 24 items and 6 sub-factors (social isolation, distancing or avoiding, discriminating, guilt, attribution, and lack of medical support) (Table 1).
The result of evaluating the fit of the structural equation model consisting 24 items and 6 factors was shown as χ2=539.72 (p<.001), Q (χ2/df)=2.28, GFI=.84, AGFI=.81, NFI=.80, TLI=.86 RMR=.03, and RMSEA=.07. While Q value was 3.0 or less, RMR was .05 or less and RMSEA was .05 to .08 so that they met the recommended level, the remaining indexes did not meet the .90 or greater which is the recommended level.
Factors were named depending on the prime item of loading to the factor (Table 1). Factor 1. Social isolation subscale: The first factor comprised five items with loadings ranging from .81~.68. Item No. 11 was "I have lost friends by telling them I have cancer." Item No. 13 was "People have physically backed away from me." Factor 2. Distancing or avoiding subscale: The second factor comprised four items with loadings ranging from .76~.68. Item No. 17 was "People avoid touching me if they know I have cancer." Item No. 19 was "Some people who know have grown more distant." Factor 3. Discrimination subscale: The third factor comprised four items with loadings ranging from .78~.57. Item No. 22 was "People with cancer are treated like outcasts." Item No. 21 was "I worry about people discriminating against me." Factor 4. Guilt subscale: The fourth factor comprised five items with loadings ranging from .77~.48. Item No. 3 was "Having cancer makes me feel like I'm a bad person." Item No. 1 was "I feel guilty because I have cancer." Factor 5. Attribution subscale: The fifth factor comprised three items with loadings ranging from .76~.73. Item No. 27 was "Cancer is viewed as a self-inflicted disease." Item No. 29 was "Others assume that cancer was caused by the patient's bad habits, even if he or she never had those habits." Factor 6. Lack of medical support subscale: The sixth factor comprised three items with loadings ranging from .89~.43. Item No. 9 was "My cancer diagnosis was delayed because my health care provider did not take my symptoms seriously." Item No. 30 was "Healthcare providers don't take 'cancer patients' signs' seriously (i.e., coughing for lung cancer, constipation for colon cancer, lumps for breast cancer, abnormal bleeding for cervix cancer, and gastric fullness and indigestion for stomach cancer)."
In this study, multi-trait multi-item matrix analysis was carried out in order to test convergent and discriminant validity of KCSS items. The result showed that the item–subscale correlation corrected for overlap for coefficients ranged from .41 to .69 so that all were .40 or greater. The success rate of the convergent validity of the item was 100%. Also in item discriminant validity, the item-own subscale correlations were higher than the item-other subscale correlations, and most item-own subscale correlations exceeded item-other subscale correlations by 2 times the standard error of correlation coefficients except for items 29 and 8. The scaling success rate was calculated by dividing the number of the item-other subscale correlations by more than 2 times of the standard error of the correlation coefficients by the total number of the item-other subscale correlations according to the method of Fayers and Machin [23], resulting in 97.5% (Table 2). And also, Cronbach's alpha coefficients of each subscale were higher than the correlation coefficients among the other subscales. Thus, it was confirmed that the properties of each subscale were discriminated [28] (Table 3).
Concurrent validity was assessed by examining the relationship of KCSS with the QOL scale (EORTC QLQ-C30). KCSS has a statistically significant negative correlation with global and functional subscales of the QLQ-C30 respectively (r=-.44; r=-.19), and KCSS has a statistically significant positive correlation with symptom subscale of the QLQ-C30 (r=.42) (Table 4).
To test the known-group comparisons, the PSI subscales (insomnia, anxiety, and depression) were categorized by two groups regarding cut-off score (4 points). For each psychological symptom, severity and interference's score were classified into distress group with 4 points or more and non-distress group with less than 4 points. In insomnia, anxiety, and depression domains, the distress group had a significantly higher perceived cancer stigma than the non-distress group, respectively (t=2.28, p=.024; t=4.63, p<.001; t=4.43, p<.001) (Table 5).
The KCSS score of patients with colorectal cancer was highest among the five types of cancer; however, there were no differences from the KCSS scores between the five types of cancer patients (colorectal cancer 38.20±11.30, breast cancer 33.94±7.71, lung cancer 35.55±9.16, cervix cancer 35.28±8.40, gastric cancer 33.76±8.00; F=1.96, p>.999).
Cronbach's alpha for the KCSS with 24 items was .89; for the six subscales, and ranged from .62~.86 (Table 1).
KCSS was developed to measure the cancer-related stigma that can be experienced in the illness trajectory process of cancer patients. The findings in this study provide a primary basis for the reliability and validity that the tool, through verifications of Korean version KCSS such as content validity, construct validity, item convergent and discriminant validity, concurrent validity, known-group validity, and reliability. As a result, the six factors that emerged in this analysis were reflected in the six subscales: social isolation, distancing or avoiding, discrimination, guilt, attribution, and lack of medical support.
The reliability of four subscales of the original version of the CLCSS for lung cancer as reported was that the Cronbach's alpha coefficient was .96 for 31-item instrument and ranged from .75 to .97 [13]. In this study, the Cronbach's alpha coefficient was .89 for the overall instrument and ranged from .62 to .86 for each subscale. Thus, the reliability of the measured variable satisfied the standard of ≥.70 [29] except for attribution (.64) and lack of medical support (.62) subscales. These results were similar to those of Chinese version of the CLCSS [15] which reported Cronbach's alpha coefficients between .60 and .88 for lung cancer patients. As Cronbach's alpha coefficients for the overall instrument were shown to be relatively high and above the standard of .70 [29], this result ensures the reliability of the instrument. However, the attribution subscale and the lack of medical support subscale among the KCSS sub-factors should be reaffirmed through a test-retest reliability verification study in the future.
In the two studies using CLCSS that were intended for lung cancer patients, the same four factors were extracted [1315]. In the original measure for lung cancer, the four factors were stigma and shame, social isolation, discrimination, and smoking status by EFA, explaining 57.0% of the total variance; the final version comprised 31 items [13]. The findings of Yang et al. [15] study with Chinese lung cancer patients were the same as the results reported by Cataldo et al. [13] and the amount of total variance explained was 58.6% from the four factors extracted by EFA. In this study, the total variance explained was 65.7% so that it was higher than the total variance of the original CLCSS and Chinese version CLCSS. Therefore, KCSS is determined as a very useful measure of stigma for Korean cancer patients. However, as the KCSS was made with modified terms of the items to apply it to five types of cancer patients whereas the CLCSS targets lung cancer patients only, there may be partly limitation of validity.
The item distribution by subscale of 24-item KCSS and item distribution of original 31-item CLCSS are to be reviewed. Guilt subscale and lack of medical support subscale items of KCSS were stigma and shame subscale in the original CLCSS, social isolation subscale and distancing or avoiding subscale items of KCSS were social isolation subscale in the original CLCSS, discrimination subscale items of KCSS were discrimination subscale in the original CLCSS and attribution subscale items of KCSS were smoking status subscales in the original CLCSS so that the similar item distributions were seen. That is, the original CLCSS has four subscales and the KCSS has six subscales so that the subscale might be further subdivided and the item distribution was similar with item distribution by subscales in original CLCSS. However, for the lack of medical support which is the sixth subscale of KCSS, item 9 was located in the smoking status of the original CLCSS and item 30 and 8 were located in the stigma and shame of the original CLCSS. This means that the factor structure can be changed for each survey that varies by target group and sample number and especially, as "lung cancer" is changed to 'cancer' for all sentences in the study, it could be because "smoking status" subscale of original CLCSS disappeared and several wordings were modified to use with the five types of cancer.
For this study construct validity, item convergent and discriminant validity, concurrent validity, and known-group validity were used as methods of testing validity. To test construct validity, χ2, Q (χ2/df), GFI, AGFI, NFI, TLI, RMR, and RMSEA were calculated through CFA. The χ2 assesses whether the actual data corresponds to the model in CFA. However, when the sample size increases, it generally becomes significant. Thus, many other suitability indicators in addition to χ2 were examined. Q, RMR and RMSEA met the recommended level among the fitness index, but the GFI, AGFI, NFI, and TLI didn't meet .90, the recommended level of the best-fit model in this study. It is not desirable in the evaluation of CFA result model for construct validity to judge the model by relying only on a single fit index and several fit indexes should be considered at the same time [21]. Therefore, in this study, a measurement model consisting of 24 items and 6 subscales was accepted in the end. However, there was the result seen that the validity of the CFA result model for construct validity did not meet some criteria in this study. Therefore, in order to establish stability of construct validity, re-test should be done through follow-up study.
In this study, the results of verifying item convergent and discriminant validity by multi-trait multi-items matrix analysis have showed 100% convergent validity and 97.5% discriminant validity of KCSS items. Items 29 and 8 were within the critical value of other subscales' items. This may mean that items 29 and 8 could be affected or confounded by other subscales. However, Cronbach's alpha coefficient of each subscale was higher than the correlation coefficients among the other subscales in this study, so that each subscale properties were discriminated [28]. Therefore, it was shown that the measurement items consistently measure construct concept and independent among subscales was maintained.
In order to verify the concurrent validity, this study utilized the QLQ-C30 questionnaire to assess the 'cancer-related quality of life" variables. In this study, significant correlations were found between the 24-item KCSS and subscales of the QLQ-C30. However, the range of correlation coefficient for the functional subscale of the QLQ-C30 and for 24-item KCSS was -.19, falling short of the recommended range for correlation coefficients (r=.40 to r=.80) [30] to establish concurrent validity. Such a low correlation is presumed to be because the participants of this study were those who were in aggressive treatment and about 69% of them are currently on anticancer chemotherapy so that it seems that there could be some limit for the stigma caused by cancer to influence the functional level of the participant compared to the global health status and treatment-related symptoms.
In order to test the known-group comparisons, we used two methods: comparison methods of the KCSS scores between (1) five types of cancer sites, and (2) the distress and non-distress groups of the PSI scores. The KCSS score of patients with colorectal cancer patients was the highest; lung, cervix, breast, and gastric cancer followed in order. However, there were statistically no differences from the KCSS scores between the five types of cancer patients. Therefore, the 24-item KCSS could potentially be applied to Korean cancer patients. As HRS that the cancer patients experience in the process of cancer treatment and its recovery means rejection, blame, or devaluation due to his or her illness [910], HRS related to the illness may increase the degree of psychological distress in cancer patients [5], it is considered that psychological distress is proper research variable for the known-group validity verification of KCSS. Hence, a significant difference in the stigma scores of the groups with different psychological distress levels establishes the known-group validity of the KCSS. This research analyzed the differences in the KCSS mean scores for each group according to the distress and non-distress groups of PSI scores. In this study, the KCSS score had a statistically significant difference between the distress and non-distress group for the PSI scores; the distress groups of insomnia, anxiety, and depression domains had a significantly higher perceived cancer stigma than the non-distress groups. Thus, the known-group validity of the KCSS instrument was established.
However, the limitation of this research is that it did not test responsiveness, which evaluates the change in degree of patientreported cancer stigma over time. Therefore, there is a need to test the responsiveness through a future longitudinal study. And also, as this study investigated cancer patients in only one city, it is suggested that further studies should be conducted on cancer patients in various regions in order to generalize KCSS in clinical practice. Lastly, as KCSS is a tool to be applied to the five types of cancer patients, we suggest the necessity of cancer-specific or sensitive stigma tool development in the future.
Cancer stigma is a very important psychological concept that cancer patients can experience during diagnosis and treatment and it affects patients' daily lives so that it is very important to objectively evaluate the cancer stigma perceived by patients in the clinical fields. Therefore, this study was conducted to test the reliability and validity of KCSS. The study result has shown that the 24-item KCSS has a relatively acceptable reliability and validity. However, in the CFA for construct validity, the fit of the model did not meet some criteria and there were two subscales (attribution subscale and lack of medical support subscale) which had relatively low Cronbach's alphas. Therefore, if these are complemented by further studies, this tool will provide a useful instrument in clinical trials investigating stigma, as well as its impact on the quality of life and psychosocial distress in Korean cancer patients.
Figures and Tables
Table 1
Factor Loading of the KCSS with Principal Component Factor Analysis with Varimax Rotation

Table 2
Multi-Trait Multi-Item Matrix (Correlation Matrix Corrected for Overlap) for Convergent and Discriminant Validity of KCSS Items

Table 3
Reliability Coefficients and Inter-subscale Correlations

Table 4
Correlations between 24-item KCSS and Subscales of QLQ-C30 for Concurrent Validity
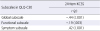
| Subscales in QLQ-C30 | 24-item KCSS |
|---|---|
| r (p) | |
| Global subscale | -.44 (<.001) |
| Functional subscale | -.19 (.003) |
| Symptom subscale | .42 (<.001) |
Table 5
Known-group Comparisons of 24-item KCSS Scores between Distress and Non-distress Groups of PSI

References
1. Ministry of Health & Welfare, Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2013. Seoul: Ministry of Health & Welfare;2015.
2. American Cancer Society. Cancer facts & figures 2016 [Internet]. Atlanta, GA: Author;2016. cited 2016 December 8. Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf.
3. National Cancer Information Center. Recommendations for distress management in cancer patients [Internet]. Seoul: Ministry for Health, Welfare and Family Affairs;2008. cited 2012 May 20. Available from: http://www.cancer.go.kr/ebook/6/PC/6.html.
4. Cho J, Choi EK, Kim SY, Shin DW, Cho BL, Kim CH, et al. Association between cancer stigma and depression among cancer survivors: A nationwide survey in Korea. Psychooncology. 2013; 22(10):2372–2378. DOI: 10.1002/pon.3302.
5. Phelan SM, Griffin JM, Jackson GL, Zafar SY, Hellerstedt W, Stahre M, et al. Stigma, perceived blame, self-blame, and depressive symptoms in men with colorectal cancer. Psychooncology. 2013; 22(1):65–73. DOI: 10.1002/pon.2048.
6. Pineles SL, Street AE, Koenen KC. The differential relationships of shame–proneness and guilt–proneness to psychological and somatization symptoms. J Soc Clin Psychol. 2006; 25(6):688–704. DOI: 10.1521/jscp.2006.25.6.688.
7. Lee I, Lee E. Concept analysis of stigma. J Korean Rheum Assoc. 2006; 13(1):53–66.
8. Lee JL, Kim KS. The relationships between stigma, distress, and quality of life in patients with lung cancer. J Korean Oncol Nurs. 2011; 11(3):237–246. DOI: 10.5388/jkon.2011.11.3.237.
9. Van Brakel WH. Measuring health-related stigma; A literature review. Psychol Health Med. 2006; 11(3):307–334. DOI: 10.1080/13548500600595160.
10. Major B, O'Brien LT. The social psychology of stigma. Annu Rev Psychol. 2005; 56:393–421. DOI: 10.1146/annurev.psych.56.091103.070137.
11. Shen MJ, Coups EJ, Li Y, Holland JC, Hamann HA, Ostroff JS. The role of posttraumatic growth and timing of quitting smoking as moderators of the relationship between stigma and psychological distress among lung cancer survivors who are former smokers. Psychooncology. 2015; 24(6):683–690. DOI: 10.1002/pon.3711.
12. Else-Quest NM, LoConte NK, Schiller JH, Hyde JS. Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychol Health. 2009; 24(8):949–964. DOI: 10.1080/08870440802074664.
13. Cataldo JK, Slaughter R, Jahan TM, Pongquan VL, Hwang WJ. Measuring stigma in people with lung cancer: Psychometric testing of the cataldo lung cancer stigma scale. Oncol Nurs Forum. 2011; 38(1):E46–E54. DOI: 10.1188/11.onf.e46-e54.
14. Chambers SK, Baade P, Youl P, Aitken J, Occhipinti S, Vinod S, et al. Psychological distress and quality of life in lung cancer: The role of health-related stigma, illness appraisals and social constraints. Psychooncology. 2015; 24(11):1569–1577. DOI: 10.1002/pon.3829.
15. Yang QQ, Liu HX, Yang CL, Ji SY, Li L. Reliability and validity of Chinese version of cataldo lung cancer stigma scale. Int J Nurs Sci. 2014; 1(1):23–27. DOI: 10.1016/j.ijnss.2014.02.011.
16. Coates C. The evolution of measuring caring: Moving toward construct validity. In : Watson J, editor. Assessing and measuring caring in nursing and health sciences. 2nd ed. New York, NY: Springer Publishing;2009. p. 261–265.
17. World Health Organization. Process of translation and adaptation of instruments [Internet]. Geneva, CH: Author;2012. cited 2012 May 20. Available from: http://www.who.int/substance_abuse/research_tools/translation/en/.
18. Lynn MR. Determination and quantification of content validity. Nurs Res. 1986; 35(6):382–385.
19. Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004; 13(4):863–868. DOI: 10.1023/b:qure.0000021692.81214.70.
20. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ-C30 scoring manual. 3rd ed. Brussels, BE: European Organisation for Research and Treatment of Cancer;2001.
21. Kim GS. Analysis structural equation modeling. Seoul: Hannarae Publishing Co.;2010.
22. Hair JF Jr, Black WC, Babin BJ, Anderson RE. Multivariate data analysis. 7th ed. Upper Saddle River, NJ: Pearson Prentice Hall;2010. p. 578–581.
23. Fayers PM, Machin D. Quality of life: The assessment, analysis and reporting of patient-reported outcomes. 3rd ed. Chichester, UK: John Wiley & Sons;2016. p. 134–136.
24. Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980; 87(2):245–251. DOI: 10.1037/0033-2909.87.2.245.
25. Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007; 60(1):34–42. DOI: 10.1016/j.jclinepi.2006.03.012.
26. Kline RB. Principles and practice of structural equation modeling. 3rd ed. New York, NY: Guilford Press;2010. p. 54–63.
27. Kaiser G. Phase-space approach to relativistic quantum mechanics. III. Quantization, relativity, localization and gauge freedom. J Math Phys. 2016; 22(4):705. DOI: 10.1063/1.524962.
28. Ware JE Jr, Gandek B. Methods for testing data quality, scaling assumptions, and reliability: The IQOLA project approach. International quality of life assessment. J Clin Epidemiol. 1998; 51(11):945–952. DOI: 10.1016/S0895-4356(98)00085-7.
29. Nunnally JC, Bernstein IH. Psychometric theory. 3rd ed. New York, NY: McGraw-Hill;1994. p. 248.
30. Park HA. Problems and issues in developing measurement scales in nursing. J Nurs Query . 2005; 14(1):46–72.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download