Abstract
Purpose
The purpose of this study was to examine the effects of Cu/Zn SOD on reduction of hindlimb muscular atrophy induced by cisplatin in rats.
Methods
Forty-two rats were assigned to three groups; control group, Cisplatin (CDDP) group and cisplatin with Cu/Zn SOD (CDDP-SOD) group. At day 35 hindlimb muscles were dissected. Food intake, activity, withdrawal threshold, muscle weight, and Type I, II fiber cross-sectional area (CSA) of dissected muscles were measured. Relative SOD activity and expression of MHC and phosphorylated Akt, ERK were measured after dissection.
Results
Muscle weight and Type I, II fiber CSA of hindlimb muscles in the CDDP group were significantly less than the control group. Muscle weight and Type I, II fiber CSA of hindlimb muscles, food intake, activity, and withdrawal thresholds of the CDDP-SOD group were significantly greater than the CDDP group. There were no significant differences in relative SOD activities of hindlimb muscles between the CDDP-SOD and CDDP groups. MHC expression and phosphorylated Akt, ERK of hindlimb muscles in the CDDP-SOD group were significantly greater than the CDDP group.
Figures and Tables
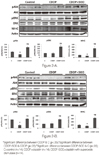 | Figure 2A. Expression of phosphorylated Akt, phosphorylated ERK and expression of MHC of soleus in C, CDDP and CDDP-SOD rats. B. Expression of phosphorylated Akt, phosphorylated ERK and expression of MHC of plantaris in C, CDDP and CDDP-SOD rats. |
References
1. Decatris MP, Sundar S, O'Byrne KJ. Platinum-based chemotherapy in metastatic breast cancer: Current status. Cancer Treat Rev. 2004; 30(1):53–81. http://dx.doi.org/10.1016/s0305-7372(03)00139-7.
2. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel). 2010; 2(11):2490–2518. http://dx.doi.org/10.3390/toxins2112490.
3. Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003; 182(1):12–20.
4. Yakabi K, Sadakane C, Noguchi M, Ohno S, Ro S, Chinen K, et al. Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology. 2010; 151(8):3773–3782. http://dx.doi.org/10.1210/en.2010-0061.
5. Gill JS, Windebank AJ. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest. 1998; 101(12):2842–2850. http://dx.doi.org/10.1172/jci1130.
6. Yoo YS, Cho OH. Relationship between quality of life and nurse-led bedside symptom evaluations in patients with chemotherapy-induced peripheral neuropathy. Asian Nurs Res. 2014; 8(1):36–41. http://dx.doi.org/10.1016/j.anr.2013.11.002.
7. Yang GS, Choe MA. Effect of anorexia and neuropathic pain induced by cisplatin on hindlimb muscles of rat. J Korean Acad Nurs. 2013; 43(3):361–369. http://dx.doi.org/10.4040/jkan.2013.43.3.361.
8. Powers SK, Smuder AJ, Judge AR. Oxidative stress and disuse muscle atrophy: Cause or consequence? Curr Opin Clin Nutr Metab Care. 2012; 15(3):240–245. http://dx.doi.org/10.1097/MCO.0b013e328352b4c2.
9. Vera G, Castillo M, Cabezos PA, Chiarlone A, Martín MI, Gori A, et al. Enteric neuropathy evoked by repeated cisplatin in the rat. Neurogastroenterol Motil. 2011; 23(4):370–378. http://dx.doi.org/10.1111/j.1365-2982.2011.01674.x.
10. Kim JI, Choe MA. A comparison between effects of anorexia induced by consecutive low-dose cisplatin and high-dose cisplatin on hindlimb muscles of rats. J Korean Biol Nurs Sci. 2012; 14(1):49–56.
11. Choe MA, An GJ, Koo BS, Jeon S. Effect of DHEA on recovery of muscle atrophy induced by parkinson's disease. J Korean Acad Nurs. 2011; 41(6):834–842. http://dx.doi.org/10.4040/jkan.2011.41.6.834.
12. Castaneda C. Muscle wasting and protein metabolism. J Anim Sci. 2002; 80:Suppl 2. E98–E105.
13. Hart PJ, Balbirnie MM, Ogihara NL, Nersissian AM, Weiss MS, Valentine JS, et al. A structure-based mechanism for copper-zinc superoxide dismutase. Biochemistry. 1999; 38(7):2167–2178. http://dx.doi.org/10.1021/bi982284u.
14. Kim W, Kim DW, Yoo DY, Chung JY, Hwang IK, Won MH, et al. Neuroprotective effects of PEP-1-Cu,Zn-SOD against ischemic neuronal damage in the rabbit spinal cord. Neurochem Res. 2012; 37(2):307–313. http://dx.doi.org/10.1007/s11064-011-0613-0.
15. Noshita N, Sugawara T, Lewen A, Hayashi T, Chan PH. Copper-zinc superoxide dismutase affects Akt activation after transient focal cerebral ischemia in mice. Stroke. 2003; 34(6):1513–1518. http://dx.doi.org/10.1161/01.str.0000072986.46924.f4.
16. Widegren U, Ryder JW, Zierath JR. Mitogen-activated protein kinase signal transduction in skeletal muscle: Effects of exercise and muscle contraction. Acta Physiol Scand. 2001; 172(3):227–238. http://dx.doi.org/10.1046/j.1365-201x.2001.00855.x.
17. Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001; 3(11):1009–1013. http://dx.doi.org/10.1038/ncb1101-1009.
18. Li G, Feng X, Wang S. Effects of Cu/Zn superoxide dismutase on strain injury-induced oxidative damage to skeletal muscle in rats. Physiol Res. 2005; 54(2):193–199.
19. Mead R. The design of experiments : Statistical principles for practical applications. 9th ed. Cambridge, UK: Cambridge University Press;1988. p. 587.
20. Baillet F, Housset M, Michelson AM, Puget K. Treatment of radiofibrosis with liposomal superoxide dismutase. Preliminary results of 50 cases. Free Radic Res Commun. 1986; 1(6):387–394.
21. Fanzani A, Zanola A, Rovetta F, Rossi S, Aleo MF. Cisplatin triggers atrophy of skeletal C2C12 myotubes via impairment of Akt signalling pathway and subsequent increment activity of proteasome and autophagy systems. Toxicol Appl Pharmacol. 2011; 250(3):312–321. http://dx.doi.org/10.1016/j.taap.2010.11.003.
22. Caso G, Garlick PJ, Ballou LM, Vosswinkel JA, Gelato MC, McNurlan MA. The increase in human muscle protein synthesis induced by food intake is similar when assessed with the constant infusion and flooding techniques. J Nutr. 2006; 136(6):1504–1510.
23. Munoz KA, Aannestad A, Tischler ME, Henriksen EJ. Skeletal muscle protein content and synthesis after voluntary running and subsequent unweighting. Metabolism. 1994; 43(8):994–999.
24. Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002; 127(1):183–198.
25. Tiidus PM, Bombardier E, Hidiroglou N, Madere R. Estrogen administration, postexercise tissue oxidative stress and vitamin C status in male rats. Can J Physiol Pharmacol. 1998; 76(10-11):952–960.
26. Rush JW, Green HJ, Maclean DA, Code LM. Oxidative stress and nitric oxide synthase in skeletal muscles of rats with post-infarction, compensated chronic heart failure. Acta Physiol Scand. 2005; 185(3):211–218. http://dx.doi.org/10.1111/j.1365-201X.2005.01479.x.
27. Tominaga T, Hachiya M, Shibata T, Sakamoto Y, Taki K, Akashi M. Exogenously-added copper/zinc superoxide dismutase rescues damage of endothelial cells from lethal irradiation. J Clin Biochem Nutr. 2012; 50(1):78–83. http://dx.doi.org/10.3164/jcbn.11-15.
28. Zhang Y, Davis C, Sakellariou GK, Shi Y, Kayani AC, Pulliam D, et al. CuZnSOD gene deletion targeted to skeletal muscle leads to loss of contractile force but does not cause muscle atrophy in adult mice. FASEB J. 2013; 27(9):3536–3548. http://dx.doi.org/10.1096/fj.13-228130.
29. Salganik RI. The benefits and hazards of antioxidants: Controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr. 2001; 20:5 Suppl. 464S–472S.
30. Akan Z, Garip AI. Antioxidants may protect cancer cells from apoptosis signals and enhance cell viability. Asian Pac J Cancer Prev. 2013; 14(8):4611–4614.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download