Abstract
Purpose
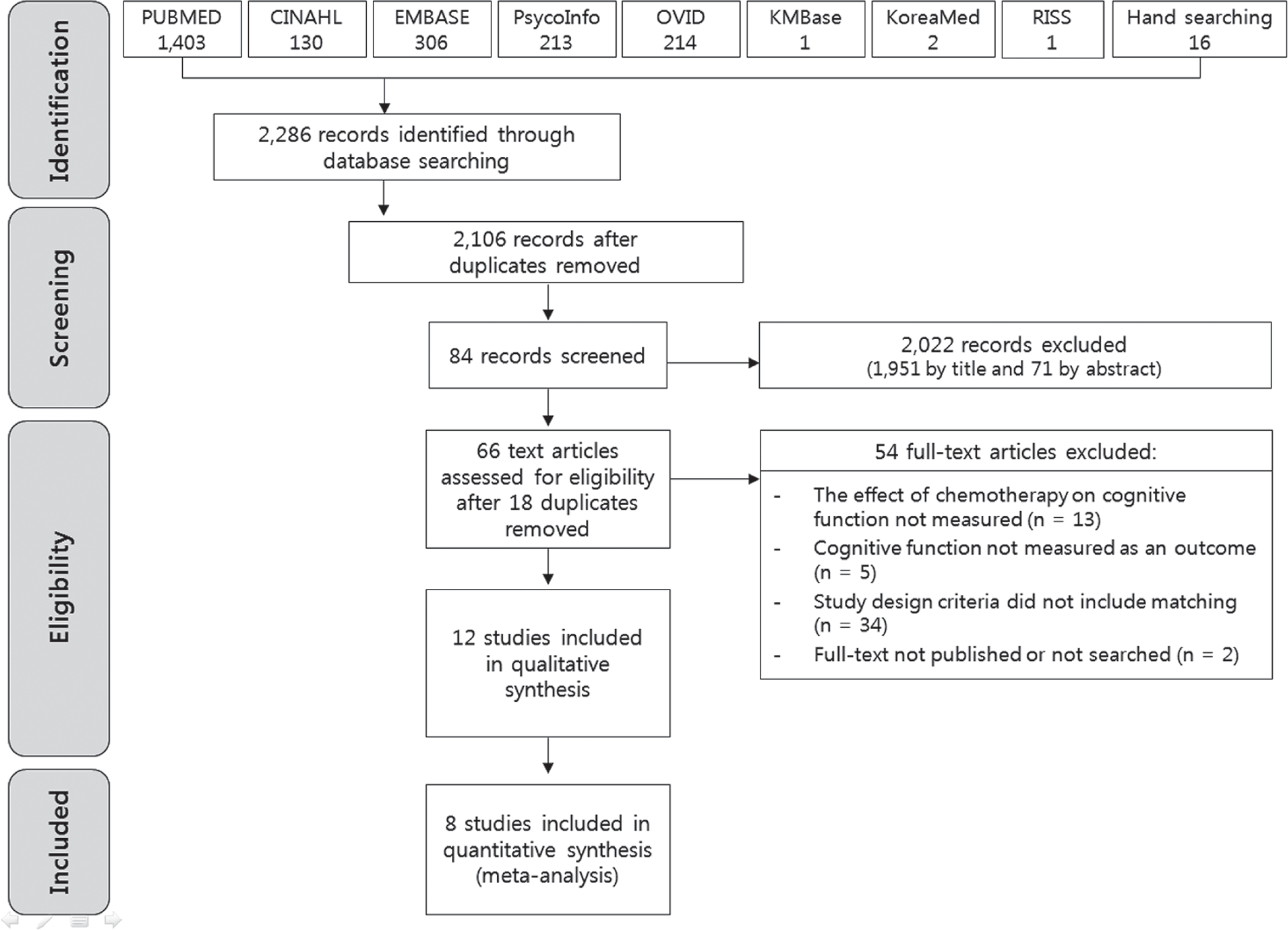
Methods
Results
References
Table 1
* AC=Doxorubicin and cyclophosphamide; ACt=Doxorubicin, cyclophosphamide, and taxane; CAF=Cyclophosphamide, doxorubicin, and 5-fluorouracil; CEA=Cyclophosphamide, epirubicin, and adriamycin; CEF=Cyclophosphamide, epirubicin, and 5-fluorouracil; CMF=Cyclophosphamide, methotrexate, and 5-fluorouracil; EC=Epirubicin and cyclophosphamide; FAC=5-fluorouracil, doxorubicin, and cyclophosphamide; FEA=5-fluorouracil, epirubicin, and adriamycin; FEC=5-fluorouracil, epirubicin, and cyclophosphamide; LH-RH=Luteinizing hormone-releasing hormone; TAC=Taxotere, adriamycin, and cyclophosphamide.
†AVRT=Auditory verbal learning test; COWAT=Controlled oral word association test; CPT=Continuous performance test; CVLT=California verbal learning test; DKEFS=Delis-Kaplan executive function system; PASAT=Paced auditory serial addition test; RAVLT=Rey auditory verbal learning test; RCFT=Rey complex figure test; RVLT=Rey visual learning test; WAIS=Wechsler adult intelligence scale; WMS-III=Wechsler memory scale-version 3; WMS-R=Wechsler memory scale-revised.
‡BDI-II=Beck depression inventory-version 2; CES-D=Center for epidemiological study–depression; CFQ=Cognitive failures questionnaire; EORTC QLQ-C30=European organization for the research and treatment of cancer quality of life questionnaire; FACT-B=Functional assessment of cancer therapy scale - for breast cancer; FACT-ES=Functional assessment of cancer therapy scale – endocrine symptom; FACT-F=Functional assessment of cancer therapy scale – fatigue; FACT-G=Functional assessment of cancer therapy scale - general; FSI=Fatigue symptom inventory; GHQ12=General health questionnaire 12; GPS=General perceived self-efficacy; HADS=Hospital anxiety and depression scale; HSCS=High sensitivity cognitive screen; MASRQ=Multiple ability self-report questionnaire; PAOF=Patient’s assessment of own functioning; POMS=Profile of mood states; PSI=Pittsburgh sleep inventory; PSS=Perceived stress scale; SCF=Subjective cognitive functioning; SLS=Satisfaction with life scale; SSQt=Social support questionnaire of transactions; STAI=State trait anxiety inventory; ZAS=Zung anxiety scale.
§Quality assessment of literature by Scottish Intercollegiate Guidelines Network checklist. ‘+ +’ indicates that all or most of the criteria have been fulfilled. ‘+’ indicates that some of the criteria have been fulfilled. ‘-’ indicates fe or no criteria fulfilled.
Abbreviation: BC=Breast cancer; CCTx=Comparison group who matched with breast cancer treated with chemotherapy; CnonCTx=Comparison group who matched with breast cancer not treated with chemotherapy; CTx=Treated with chemotherapy; DCIS=Ductal carcinoma in situ; HRTx=Treated with hormone therapy; M=mean; nonCTx=Not treated with chemotherapy; RTx=Treated with radiotherapy; SD=Standard deviation; U.K.=United Kingdom; U.S.A.=United States of America.
Table 2.
Table 3.
| Test | No. of studies* | n† | Effect size‡ | Lower 95% CI | Upper 95% CI | Z | p | Nfs |
|---|---|---|---|---|---|---|---|---|
| Attention/concentration | 19 | 1,515 | −0.14 | −0.24 | −0.03 | 2.57 | .010 | 15 |
| Digit span-forward and backward | 3 | 265 | −0.08 | −0.32 | 0.16 | 0.64 | .524 | |
| Digit span-forward | 2 | 127 | −0.24 | −0.73 | 0.25 | 0.96 | .337 | |
| Trails making test-part A | 5 | 392 | −0.06 | −0.27 | 0.14 | 0.61 | .538 | |
| Arithmetic | 4 | 311 | −0.06 | −0.29 | 0.17 | 0.49 | .626 | |
| Letter-number sequencing | 5 | 420 | −0.28 | −0.48 | −0.08 | 2.78 | .005 | |
| Memory | 30 | 2,204 | −0.17 | −0.25 | −0.08 | 3.66 | <.001 | 73 |
| Verbal memory | 20 | 1,602 | −0.17 | −0.28 | −0.07 | 3.19 | .001 | 32 |
| CVLT short delay free recall | 3 | 265 | −0.39 | −0.64 | −0.14 | 3.11 | .002 | |
| CVLT long delay free recall | 3 | 265 | −0.08 | −0.33 | 0.16 | 0.68 | .500 | |
| CVLT long delay recognition | 3 | 265 | -0.06 | -0.30 | 0.19 | 0.47 | .636 | |
| RAVLT immediate recall | 6 | 458 | −0.21 | −0.41 | 0.00 | 1.94 | .052 | |
| RAVLT long delay recall | 5 | 349 | −0.11 | −0.36 | 0.14 | 0.86 | .392 | |
| Visual memory | 10 | 602 | −0.15 | −0.32 | 0.01 | 1.85 | .063 | 0 |
| RCFT immediate recall | 5 | 301 | −0.19 | −0.42 | 0.04 | 1.59 | .113 | |
| RCFT long delay recall | 5 | 301 | −0.12 | −0.35 | 0.11 | 1.04 | .297 | |
| Executive function | 17 | 1,407 | -0.08 | -0.18 | 0.03 | 1.35 | .176 | 0 |
| Digit symbol–coding subset | 4 | 311 | −0.09 | −0.32 | 0.14 | 0.76 | .444 | |
| Digit symbol-symbol search subset | 4 | 311 | −0.09 | −0.32 | 0.14 | 0.80 | .421 | |
| Trails making test-part B | 5 | 392 | −0.09 | −0.30 | 0.11 | 0.90 | .366 | |
| Digit span-backward | 4 | 393 | −0.03 | −0.24 | 0.19 | 0.24 | .812 | |
| Visuospatial skill | 2 | 127 | −0.11 | −0.47 | 0.26 | 0.58 | .562 | − |
| RCFT copy | 2 | 127 | -0.11 | -0.47 | 0.26 | 0.58 | .562 | |
| Language | 3 | 265 | −0.02 | −0.26 | 0.23 | 0.13 | .902 | 0 |
| Boston naming test | 3 | 265 | −0.02 | −0.26 | 0.23 | 0.13 | .902 | |
| Subjective cognitive function | 6 | 474 | −0.26 | −0.44 | −0.07 | 2.76 | .006 | 6 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download