Abstract
Purpose
The purpose of this study was to determine the effect of dehydroepiandrosterone (DHEA) on recovery of muscle atrophy induced by Parkinson's disease.
Methods
The rat model was established by direct injection of 6-hydroxydopamine (6-OHDA, 20 µg) into the left striatum using stereotaxic surgery. Rats were divided into two groups; the Parkinson's disease group with vehicle treatment (Vehicle; n=12) or DHEA treatment group (DHEA; n=22). DHEA or vehicle was administrated intraperitoneally daily at a dose of 0.34 mmol/kg for 21 days. At 22-days after DHEA treatment, soleus, plantaris, and striatum were dissected.
Results
The DHEA group showed significant increase (p<.01) in the number of tyrosine hydroxylase (TH) positive neurons in the lesioned side substantia nigra compared to the vehicle group. Weights and Type I fiber cross-sectional areas of the contralateral soleus of the DHEA group were significantly greater than those of the vehicle group (p=.02, p=.00). Moreover, extracellular signal-regulated kinase (ERK) phosphorylation significantly decreased in the lesioned striatum, but was recovered with DHEA and also in the contralateral soleus muscle, Akt and ERK phosphorylation recovered significantly and the expression level of myosin heavy chain also recovered by DHEA treatment.
Parkinson's disease (PD) is the second most common neurodegenerative disorder just behind Alzheimer and its prevalence is likely to increase due to the aging population and caused by the massive destruction of dopaminergic neurons of the substantia nigra pars compacta (SNpc) that project to the striatum thus leading to extensive loss of striatal dopamine (DA) concentrations (Hornykiewicz, 1989). PD is characterized behaviorally by impairments in gait, such as shuffling and short-stepped locomotion, low walking velocity, small angular displacement of segments, and impairments in posture and balance (Mitoma, Hayashi, Yanagisawa, & Tsukagoshi, 2000). These impairments are attributable to an inability to regulate and adjust stride length (Morris, Iansek, Matyas, & Summers, 1994), compromised mono and polysynaptic reflexes of leg extensor and flexor muscles and low maximum activity of gastrocnemius, soleus and tibialis anterior muscles (Mitoma et al.). These deficits might be compensated for by adjustments that are typical for PD, including widened stance, increased ratio of support period to stride period, exaggerated activities of the thigh and leg muscles, and low walking speed (Mitoma et al.). Recent studies using mechanical devices seem to have provided evidence that muscle strength is reduced in patients with PD compared with age-matched controls even at early stages of the disease and in the unaffected side (Cano-de-la-Cuerda, Pérez-de-Heredia, Miangolarra-Page, Muñoz-Hellín, & Fernández-de-Las-Peñas, 2010).
The human pathological features of PD can be mimicked in rats by injection of the neurotoxin 6-hydroxydopamine (6-OHDA) to induce striatal dopamine depletion (Schwarting & Huston, 1996). The injections are usually made unilaterally and so affect motor performance on the contralateral side of the body, including skilled fore- and hindlimb use (Whishaw et al., 2002) and sensorimotor functions (Muir & Whishaw, 1999). Rats with unilateral 6-OHDA lesions exhibit characteristic gait disturbances during overground locomotion such as compensatory weight support shifts to the unaffected side during propulsion and turning (Muir & Whishaw). Moreover, we recently reported that contralateral soleus muscle atrophy occurs at 21 days after establishing the PD rat model with unilateral 6-OHDA lesions (Kim & Choe, 2010), supporting that muscle atrophy is present in PD patients. However, there are few studies on the recovery of muscle atrophy induced by PD.
Dehydroepiandrosterone (DHEA) as a precursor of estradiol and testosterone represents the most abundant steroid hormone in the human body and can be synthesized de novo in the brain (Maninger, Wolkowitz, Reus, Epel, & Mellon, 2009). Levels of DHEA decrease gradually with age and this decline may be linked with different age-associated diseases (Weill-Engerer et al., 2003). In PD patients, lower DHEA levels were associated with higher ratings of psychopathology, poorer memory performance, and more severe Parkinsonian movements (Harris, Wolkowitz, & Reus, 2001). Thus, it is possible that exogenous DHEA supplementation could have therapeutic benefits (Brown et al., 1999). Moreover, DHEA administration attenuates unaffected plantaris and gastrocnemius muscle atrophy in rats with neuropathic pain induced by unilateral peripheral nerve injury (Choe & An, 2009). Unlike hormones such as testosterone and estrogen, DHEA is sold over the counter and by mail order as a nutritional supplement. Therefore, DHEA can be used as complementary and alternative therapy for nursing intervention.
The signaling pathway activated by DHEA has been reported that neuroprotective effect of DHEA is dependent on phosphatidylinositol-3 kinase (PI3-K)/protein kinase B (Akt) and extracellular signal-regulated kinase (ERK) signaling pathways in neuronal cells (Leskiewicz et al., 2008). Thus, we examined whether DHEA could recover the muscle atrophy in the PD animal model and which signaling pathway is involved in the recovery of atrophied muscle.
An experimental control group design was used for the study. Rats were assigned randomly to a DHEA and vehicle group. All rats had operation inducing Parkinson's disease. DHEA group received DHEA treatment, whereas vehicle group received normal saline administration for 21 days after operation. Bilateral soleus and plantaris muscles were dissected on day 22 of the experiment. The experimental procedures were performed in accordance with the animal care guidelines of the National Institute of Health (NIH) and carried out with a prior approval from the Institutional Animal Ethical Committee of Dongguk University (IRB No. 2009-1116).
Male Sprague-Dawley rats (Daehan Experimental Co., Korea) 170±15 g were used for the experiment. The animals were housed under laboratory conditions at a controlled temperature (20±2℃) and maintained under light-dark cycles, 12 hours of each (from 07:00 to 19:00 h). Water and pellets (Samyang Co., Cheonan, Korea) were provided ad libitum.
Rats were anesthetized with Rompun (Vial Korea, 10 mg/kg) plus Zoletil 50 (Virbac Korea, 25 mg/kg) intramuscular anesthesia and given a unilateral stereotaxic injection of 20 µg 6-OHDA (4 µg/µL) with 0.2 mg/mL L-ascorbic acid into the left striatum (AP 0.7, ML 2.6, DV 4.5; all coordinates represent millimeter adjustments from bregma) at a rate of 1 µL/min using a 26-gauge Hamilton syringe. The day after stereotaxic injection, amphetamine-induced rotation test was performed (Kim & Choe, 2010) and rats showing rotation rate more than 5 times/min were selected for DHEA or vehicle treatment (average rate is 7±2).
The body weights were measured twice a week by a rat digital balance (Daejong instruments, Seoul, Korea).
All groups were allowed to have water and pellets ad libitum. Food pellets were weighed and a consistent amount added to the food tray. Food intake was measured twice a week for 21 days and the total amount across the 21days was used in the data analysis.
Two research assistants observed the activity of all the rats at a regular time twice a week. Physical movement, grooming, and inactivity were assessed. Physical movement was rated active '1', grooming '2', and inactive '3'. Each rat was observed for 15 seconds for a total of 5 minutes (20 observations). Interrater reliability of the two research assistants was K coefficients was .87. Two observers assessed the physical activity of rats every 15 seconds for five minutes which makes 20 observations. We chose one observation out of two. Total activity score was calculated by obtaining mean activity score of 6 observations.
Rats were anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg, supplemented as required) on Day 22 following DHEA treatment. The soleus and plantaris muscles were excised bilaterally, the wet mass of individual muscles was obtained, and the tissue was rinsed with normal saline. Following muscle dissection, pentobarbital sodium was supplemented intraperitoneally to euthanize the rats. The weights of dissected individual muscles were measured using a microbalance (Mettler PE 160, Columbus, OH, USA) after fat and connective tissues were trimmed.
The method that we used for measuring the cross-sectional areas of Types I and II muscle fibers was described in Choe et al. (2004). From each muscle, a portion was cut transversely from the midsection, mounted in wooden pieces and quick frozen by immersion in isopentane cooled with liquid nitrogen. Transverse sections 10 µm thick were sectioned in a cryostat at -20℃, adhered to cover glasses, thawed, and air-dried at room temperature for 30 minutes. Myosin ATPase reactions were performed on serial sections and fibers were classified as Type I (slow-twitch) or Type II (fast-twitch) based on this reaction.
Pre-incubation was carried out for 5 minutes in a medium of both pH 4.3 and pH 4.6 acetate buffer. The medium contained 200 mM acetate, and pH was adjusted by 2M HCl. The sections were then rinsed with normal saline. Incubation was carried out for 30 minutes at 37℃ in a medium that contained 1.1 M sodium barbiturate 20 mL, 180 mM CaCl2 10 mL and 152 mg ATP. The pH was adjusted to 9.4. Sections were rinsed with normal saline following the incubation then rinsed with 1% CaCl2 once every 2 minutes for 6 minutes (i.e., a total of three times). Sections were reacted with 2% CoCl2 for 3 minutes then rinsed with normal saline five times. Sections were allowed to react with 2% ammonium sulfate for 2 minutes then rinsed with normal saline five times.
Following histochemical staining and incubations, sections were dehydrated through a series of ethanol concentrations from 70% to 100%, cleared in xylene and mounted. Type I fibers were identified as those staining dark, while Type II fibers were identified as those staining light in ATPase reactions after pre-incubation. Fiber cross-sectional area was calculated from tracings of 50-100 muscle fibers at 100× magnification by microscopic image analyzer (Leity, ASM 68k, Netzler).
The animals were sacrificed on day 22 after DHEA treatment, and were then perfused transcardially with 4% paraformaldehyde in 0.05 M phosphate buffer (PB). The brains were removed, postfixed, and cryoprotected. Immunohistochemistry was performed by using free-floating cryomicrotome-cut sections (40 mm thickness) that encompassed the entire SN. After incubation with 3% H2O2 in 0.05 M PBS, the sections were stained overnight at room temperature using an anti-TH (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) primary antibody for DA neurons. The Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) was used as the secondary antibody. For visualization of TH protein, 3,3'-Diaminobenzidine (DAB) staining method was used. Diaminobenzidine is oxidized by hydrogen peroxide in the presence of hemoglobin to give a dark-brown color.
For western blotting, the striatum and muscle tissues were homogenized in lysis buffer containing 50 mM Tris-base (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1% glycerol, 10 mM NaF, 10 mM Na-pyrophosphate, 1% NP-40 and protease inhibitors (0.1 mM phenylmethylsulfonylfluoride, 5 µg/mL aprotinin, and 5 µg/mL leupeptin). Thirty µg of cell lysates were electrophoresed in 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes which were then incubated with anti-phospho-MAPK, anti-MAPK, anti-phospho-Akt, anti-Akt (Cell signaling technology, Beverly, MA, USA), anti-MHC (Abcam, Cambridge, UK) and anti-β-actin (Sigma, St. Louis, MO, USA) for 16 h at 4℃. After washing with Tris-buffered saline with 0.05% of Tween 20 the blots were incubated with horseradish peroxidase-conjugated anti-rabbit IgG, and the bands were visualized using the ECL system (Thermo Fisher Scienctific, USA). Band images were obtained by using Molecular Imager ChemiDoc XRS+ (Bio-Rad, Hercules, CA, USA) and band intensity was analyzed by Image Lab™ software version 2.0.1 (Bio-Rad).
As shown in Table 1, there was no significant difference on body weight between DHEA and vehicle groups at the first day of the experiment (t=0.28, p=.78). Two groups demonstrated a significant increase in body weight between preexperiment and postexperiment: weight of the DHEA group increased by 97.9% (p<.01), that of the vehicle group increased by 94.0% (p<.01). There was no significant difference on body weight on 22 days following DHEA treatment between the DHEA and vehicle groups (t=0.80, p=.42).
As presented in Table 2, there was no significant difference on total dietary intake between DHEA and vehicle groups (t=0.22, p=.82).
Activity score of the DHEA and vehicle groups are shown in Table 2. There was no significant difference on total activity score between DHEA and vehicle groups (t=0.10, p=.92).
Muscle weights of the DHEA and vehicle group are shown in Table 3. The weight of right soleus muscle was 110.5% greater in the DHEA group than in the vehicle group (t=2.31, p=.02). There are no significant differences of plantaris muscles between DHEA and vehicle groups.
The effects of DHEA in 6-OHDA-injected rat were judged by immunohistochemistry of TH-positive DA neurons in the SN and immunoblot analysis of TH in the striatum. Photomicrographs of TH-positive neurons in the SN are shown in Figure 2A and B. The 6-OHDA-injected lesioned SN had significantly fewer TH positive neurons, as compared with the intact SN (p<.01). However, DHEA treated groups had significantly greater TH positive neurons in the lesioned SN, as compared with the vehicle group (p<.01, Figure 2). In addition, DHEA significantly increased TH expression level in the lesioned striatum, as compared with the vehicle group (p<.01, Figure 3).
To examine the mechanisms of action of DHEA on the recovery of TH-positive neurons, we observed phosphorylation level of Akt and ERK in the striatum, known that are activated by estrogen in the neuroblastoma cell line, SH-SY5Y (Leskiewicz et al., 2008). There was no significant difference on the Akt phosphorylation between lesioned and intact striatum. However, in the vehicle treated striatum, ERK phosphorylation decreased, but DHEA significantly recovered ERK phosphorylation (p<.01, Figure 4). Next, in order to find out the mechanisms of action of DHEA on the atrophied muscle, we examined the phosphorylation level of Akt and ERK in the soleus muscle. In the vehicle treated rat, Akt and ERK phosphorylation decreased in the contralateral soleus muscle, but DHEA recovered it (Figure 5A). Interestingly, DHEA increased the ERK phosphorylation by 2.5 fold in the contralateral soleus muscle compared with that of ipsilateral (Figure 5A). To confirm the recovery of atrophied muscle, the expression level of myosin heavy chain (MHC) was examined in the muscle. MHC isoforms have myosin adenosinetriphosphatase activities correlated with the speed of muscle fiber shortening (Bárány, 1967). Therefore, the MHC expression has been used as a phenotypic marker for functional aspects of muscle fibers and has been changed in the regenerated muscle fiber (Bigard et al., 1996). As shown in figure 5B, MHC expression decreased in the contralateral soleus muscle of vehicle treated rat, but DHEA recovered it.
In this study, we demonstrated that DHEA treatment significantly increases in the number of tyrosine hydroxylase positive neuron of the lesioned side SN and, weights and Type I fiber cross-sectional area of the contralateral soleus compared with that of vehicle group. Moreover, DHEA recovered the decreased level of Akt and ERK phosphorylation in the contralateral soleus muscle.
Rats with unilateral DA depletions (hemi-Parkinson rats) display impairments in their contralateral hindlimbs in adjusting posture and moving (Whishaw et al., 2002). They compensate by supporting themselves mainly on their ipsilateral hindlimb. Thus, their center of gravity is shifted to the ipsilateral side and movement is preferentially directed toward the ipsilateral side, in part to maintain equilibrium and in part to remove weight from the contralateral limbs so that they can enter the swing phase of the stepping cycle. It is proposed that the contralateral limbs may be unable to apply force to adjust posture and produce movement (Whishaw et al.). In addition, we also examined the decreased locomotor activity and contralateral hindlimb soleus muscle atrophy in the hemi-Parkinson rats (Kim & Choe, 2010), suggesting that hemi-Parkinson rats display impairments in locomotion activity and it results in muscle atrophy including reduction of muscle protein synthesis and stimulation of muscle protein degradation (Frimel et al., 2005). Previously, it has been reported that DHEA is able to potentiate locomotor activity of hemiparkinsonian monkeys (Bélanger, Grégoire, Bédard, & Di Paolo, 2003).
Various targets of DHEA have been reported in brain (Mehta & Ticku, 2001; Bergeron, de Montigny, & Debonnel, 1996). DHEA is a negative modulator of gamma-aminobutyric acid (GABA) A receptor that interacts with the barbiturate site of the GABAA receptor complex (Mehta & Ticku). DHEA is shown to reduce the amplitude of inhibitory postsynaptic potentials caused by GABA inputs, depresses the depolarizing responses to glutamate, and blocks the glutamate-induced action potentials (Bergeron, de Montigny, & Debonnel). An increase in synaptic concentrations of DHEA could increase neuronal excitability and CNS arousal. Thus DHEA, by their antagonistic action on GABAA receptors in basal ganglia, could possibly stimulate the output pathways of the striatum by decreasing inhibitory GABAergic activity (Mehta & Ticku). DHEA also potentiates neuronal N-methyl-d-aspartate (NMDA) (Bergeron et al.). NMDA receptors are abundant in the output structures of the basal ganglia. DHEA such as estrogens can affect various dopaminergic targets of the nigrostriatal pathways such as DA synthesis, uptake, transporters and receptors and downstream signalization, some of which are destroyed in severely impaired 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkeys (Cyr et al., 2000). It is well documented that estrogens display modulatory and neuroprotective activities on DA neurochemistry and behaviors mediated by DA (Cyr et al.). DHEA may also be beneficial in MPTP monkeys by its antiglucocorticoid activity (Kalimi, Shafagoj, Loria, Padgett, & Regelson, 1994).
An alternative mechanism of action of DHEA is to induce biologically active insulin-like growth factor-I (IGF-I) (Yen, Morales, & Khorram, 1995), which has been shown to stimulate muscle protein synthesis in humans (Fryburg, Jahn, Hill, Oliveras, & Barrett, 1995). Previously, we also examined that DHEA administration prevents steroid induced muscle atrophy (Choe & An, 2009). Although the mechanism underlying DHEA's effects on muscle metabolism remains unclear, these suggest that DHEA may affect on the weakened muscle for potentiating locomotor activity in the hemiparkinson animals. In the present study, DHEA administration recovers atrophied muscle in PD rat model. Thus, these results suggest that DHEA affects the destroyed DA affects the destroyed DA through direct or indirect manner, and strengthens directly atrophied muscle for recovery of locomotor activity.
The signaling pathway of neuroprotective effects of DHEA is poorly understood. Leskiewicz et al., (2008) reported that neuroprotective effect of DHEA is dependent on phosphatidylinositol-3 kinase (PI3-K)/Akt and ERK/MAPK signaling pathways in SH-SY5Y, neuroblastoma cells. However, in the cortical neurons, DHEA attenuates apoptotic and excitotoxic cell death through ERK/MAPK signaling pathway, but not PI3-K/Akt (Leskiewicz et al.). DHEA could be its transformation into other active steroids such as testosterone and estradiol by the converting enzymes aromatase and hydroxysteroid dehydrogenase present in the brain (Weill-Engerer et al., 2003). Estrogen has been reported to protect against striatal toxicity following 6-OHDA injection into striatum (Garcia-Segura, Azcoitia, & DonCarlos, 2001). The mechanisms of action of estrogen are associated with two important signaling pathways, the ERK and the PI3-K/Akt pathways in neuronal cells (Leskiewicz et al.). Estrogen receptors (ERs) can interact directly with the ERK pathway through sequential activation of Ras, B-raf, MAPK/ERK kinase (MEK1/2), and finally ERK1/2 (MAPK) resulting in the activation of a wide variety of transcription factors involved in neuronal survival (Mhyre & Dorsa, 2006). ERs can also interact with the PI3-K signaling pathway leading to the activation of the effector Akt (Mhyre & Dorsa). Activated Akt can modulate the expression of proteins influencing cell death, such as inhibitors of apoptosis, Bcl-2 and Bcl-x or inducers of apoptosis, Bax and Bad (Garcia-Segura et al., 2001). In this study, we demonstrated that DHEA activates the ERK pathway, but not Akt in the neuroprotection and both pathways in recovery of atrophied muscle in the 6-OHDA-induced PD model.
In the present study, DHEA recovered atrophied soleus muscle weights and Type I fiber cross-sectional area in the PD animal model. The soleus muscle is primarily made up 85% of type I fibers in rat. However, the plantaris muscle is primarily made up 90% of fast type II fibers (American College of Sports Medicine, 2006). Moreover, DHEA could not enhance adaptations associated with resistance training in young men (Brown et al., 1999), whereas, DHEA could increase muscle strength in elderly (Morales, Haubrich, Hwang, Asakura, & Yen, 1998) and in patients with myotonic dystrophy (Sugino et al., 1998). In animal study, the effect of DHEA administration was not shown increased muscle protein synthesis in normal rat (Choe & An, 2009). Based on these previous studies, DHEA may facilitate protein synthesis in patients with damaged muscles.
Our findings indicate that DHEA treatment recovers decreased weights and type I fiber cross-sectional area of the contralateral soleus as well as TH-positive cells in the 6-OHDA-induced hemi-Parkinson rat model. This study supports that DHEA may affect the weakend muscle as well as damaged brain in the Parkinson rat for relieving motor symptoms, which may also be beneficial to slow progression of PD.
Figures and Tables
Figure 1
Cross-sections of the hindlimb muscles in DHEA (left) and Vehicle (right) rats.
The first line is the left (ipsilateral) soleus in DHEA and Vehicle rats. The second line is the left plantaris in DHEA and Vehicle rats. The third line is the right (contralateral) soleus in DHEA and Vehicle rats. The fourth line is the right plantaris in DHEA and Vehicle rats. Dark=TypeI muscle fiber, light=TypeII muscle fiber (Myosin ATPase straining, 100×).
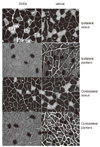
Figure 2
TH-specific immunohistochemical staining and the number of TH positive neurons in substantia nigra in unilateral 6-OHDA lesioned Parkinson rat.
The data represent the means±SEM; *Significant difference between intact side & 6-OHDA lesioned side (p<.01).
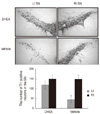
Figure 3
TH-immunoblot in the striatum in unilateral 6-OHDA lesioned Parkinson rat.
The data represent the means±SEM; *Significant difference between intact side & 6-OHDA lesioned side (p<.01).

Figure 4
DHEA induces ERK phosphorylation in the lesioned striatum.
The data represent the means±SEM; *Significant difference between intact side & 6-OHDA lesioned side (p<.01).

Figure 5
DHEA induces ERK and Akt phosphorylation and recovered MHC expression in the unaffected side of soleus of unilateral rat Parkinson's disease model.
The data represent the means±SEM; *Significant difference between DHEA & vehicle treated rat of soleus (p<.01).
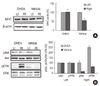
Table 2
Total Amount of Diet Intake, Weight Gain, and Total Activity Score during the Experimental Period

References
1. American College of Sports Medicine. ACSM's advanced exercise physiology. 2006. Philadelphia: Lippincott Williams & Wilkins.
2. Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. The Journal of General Physiology. 1967. 50:197–218.
3. Bélanger N, Grégoire L, Bédard P, Di Paolo T. Estradiol and dehydroepiandrosterone potentiate levodopa-induced locomotor activity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys. Endocrine. 2003. 21:97–101.
4. Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: Effects mediated via sigma receptors. Journal of Neuroscience. 1996. 16:1193–1202.
5. Bigard XA, Janmot C, Merino D, Lienhard F, Guezennec YC, D'Albis A. Endurance training affects myosin heavy chain phenotype in regenerating fast-twitch muscle. Journal of Applied Physiology. 1996. 81:2658–2665.
6. Brown GA, Vukovich MD, Sharp RL, Reifenrath TA, Parsons KA, King DS. Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. Journal of Applied Physiology. 1999. 87:2274–2283.
7. Cano-de-la-Cuerda R, Pérez-de-Heredia M, Miangolarra-Page JC, Muñoz-Hellín E, Fernández-de-Las-Peñas C. Is there muscular weakness in Parkinson's disease? American Journal of Physical Medicine and Rehabilitation. 2010. 89:70–76.
8. Choe MA, An GJ. Effect of DHEA administration alone or exercise combined with DHEA before steroid treatment on rat hindlimb muscles. Journal of Korean Academy of Nursing. 2009. 39:321–328.
9. Choe MA, An GJ, Lee YK, Im JH, Choi-Kwon S, Heitkemper M. Effect of inactivity and undernutrition after acute ischemic stroke in a rat hindlimb muscle model. Nursing Research. 2004. 53:283–292.
10. Cyr M, Calon F, Morissette M, Grandbois M, Di Paolo T, Callier S. Drugs with estrogen-like potency and brain activity: Potential therapeutic application for the CNS. Current Pharmaceutical Design. 2000. 6:1287–1312.
11. Frimel TN, Kapadia F, Gaidosh GS, Li Y, Walter GA, Vandenborne K. A model of muscle atrophy using cast immobilization in mice. Muscle and Nerve. 2005. 32:672–674.
12. Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. Journal of Clinical Investigation. 1995. 96:1722–1729.
13. Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Progress in Neurobiology. 2001. 63:29–60.
14. Harris DS, Wolkowitz OM, Reus VI. Movement disorder, memory, psychiatric symptoms and serum DHEA levels in schizophrenic and schizoaffective patients. World Journal of Biological Psychiatry. 2001. 2:99–102.
15. Hornykiewicz O. Ageing and neurotoxins as causative factors in idiopathic Parkinson’s disease-a critical analysis of the neurochemical evidence. Progress in Neuro-psychopharmacology and Biological Psychiatry. 1989. 13(3):19–328.
16. Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Molecular and Cellular Biochemistry. 1994. 131:99–104.
17. Kim Y, Choe MA. Effect of decreased locomotor activity on hindlimb muscles in a rat model of Parkinson's disease. Journal of Korean Academy of Nursing. 2010. 40:580–588.
18. Leskiewicz M, Regulska M, Budziszewska B, Jantas D, Jaworska-Feil L, Basta-Kaim A, et al. Effects of neurosteroids on hydrogen peroxide- and staurosporine-induced damage of human neuroblastoma SH-SY5Y cells. Journal of Neuroscience Research. 2008. 86:1361–1370.
19. Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Frontiers in Neuroendocrinology. 2009. 30:65–91.
20. Mehta AK, Ticku MK. Unsulfated and sulfated neurosteroids differentially modulate the binding characteristics of various radioligands of GABA(A) receptors following chronic ethanol administration. Neuropharmacology. 2001. 40:668–675.
21. Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: Role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006. 138:851–858.
22. Mitoma H, Hayashi R, Yanagisawa N, Tsukagoshi H. Characteristics of parkinsonian and ataxic gaits: A study using surface electromyograms, angular displacements and floor reaction forces. Journal of the Neurological Sciences. 2000. 174:22–39.
23. Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100mg daily dose of DHEA on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Journal of Clinical Endocrinology and Metabolism. 1998. 78:1360–1367.
24. Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain. 1994. 117:1169–1181.
25. Muir GD, Whishaw IQ. Ground reaction forces in locomoting hemi-parkinsonian rats: A definitive test for impairments and compensations. Experimental Brain Research. 1999. 126:307–314.
26. Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Progress in Neurobiology. 1996. 50:275–331.
27. Sugino M, Ohsawa N, Ito T, Ishida S, Yamasaki H, Kimura F, Shinoda K. A pilot study of dehydroepiandrosterone sulfate in myotonic dystrophy. Neurology. 1998. 51:586–589.
28. Weill-Engerer S, David JP, Sazdovitch V, Liere P, Schumacher M, Delacourte A, et al. In vitro metabolism of dehydroepiandrosterone (DHEA) to 7alpha-hydroxy-DHEA and delta5-androstene-3beta, 17beta-diol in specific regions of the aging brain from Alzheimer's and non-demented patients. Brain Research. 2003. 969:117–125.
29. Whishaw IQ, Suchowersky O, Davis L, Sarna J, Metz GA, Pellis SM. Impairment of pronation, supination, and body co-ordination in reach-to-grasp tasks in human Parkinson's disease (PD) reveals homology to deficits in animal models. Behavioural Brain Research. 2002. 133:165–176.
30. Yen S, Morales A, Khorram O. Replacement of DHEA in aging men and women: Potential remedial effects. Annals of the New York Academy of Sciences. 1995. 774:128–142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download