Abstract
Background
Oxidative stress and inflammation are important factors in the pathogenesis of diabetes and contribute to the development of diabetic complications. To understand the mechanisms that cause vascular complications in diabetes, we examined the effects of high glucose and/or free fatty acids on the production of superoxide from neutrophils and their role in endothelial cell damage.
Methods
Human neutrophils were incubated in the media containing 5.5 mM D-glucose, 30 mM D-glucose, 3 nM oleic acid, or 30 µM oleic acid for 1 hour to evaluate superoxide production through NAD(P)H oxidase activation. Human aortic endothelial cells were co-cultured with neutrophils exposed to high glucose and oleic acid. We then measured neutrophil adhesion to endothelial cells, neutrophil activation and superoxide production, neutrophil-mediated endothelial cell cytotoxicity and subunits of neutrophil NAD(P)H oxidase.
Results
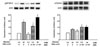
After 1 hour of incubation with various concentrations of glucose and oleic acid, neutrophil adherence to high glucose and oleic acid-treated endothelial cells was significantly increased compared with adhesion to low glucose and oleic acid-treated endothelial cells. Incubation of neutrophils with glucose and free fatty acids increased superoxide production in a dose-dependent manner. High glucose and oleic acid treatment significantly increased expression of the membrane components of NAD(P)H oxidase of neutrophil (gp91phox). Endothelial cells co-cultured with neutrophils exposed to high glucose and oleic acid showed increased cytolysis, which could be prevented by an antioxidant, N-acetylcysteine.
Figures and Tables
 | Fig. 1Effect of high glucose and/or oleic acid on neutrophil adhesion to endothelial cells. *P < 0.01; †P < 0.01; ‡P < 0.001 vs. cells treated with normal glucose or low oleic acid for 1 h. §P < 0.001 vs. cells treated with high glucose and high oleic acid. NAC, N-acetylcystein. |
 | Fig. 2Effect of high glucose and/or oleic acid on neutrophil activation. *P < 0.01; †P < 0.01; ‡P < 0.001 vs. cells treated with normal glucose or low oleic acid for 1 h. |
 | Fig. 3Effect of high glucose and/or oleic acid on neutrophil superoxide production. *P < 0.01; †P < 0.01; ‡P < 0.001 vs. cells treated with normal glucose or low oleic acid for 1 h. §P < 0.001 vs. cells treated with high glucose and high oleic acid. NAC, N-acetylcystein. |
References
1. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991. 14:173–194.

2. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complication in insulin-dependent diabetes mellitus. N Eng J Med. 1993. 329:977–986.
3. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000. 87:1123–1132.

4. Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988. 256:205–212.

5. Schmidt AM, Hori O, Brett J, Yan SD, Wautler JL, Stern D. Cellular receptors for advanced glycation end products: implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994. 14:1521–1528.

6. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999. 340:115–126.
7. Shurtz-Swirski R, Sela S, Herskovits AT, Shasha SM, Shapiro G, Nasser L, Kristal B. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care. 2001. 24:104–110.

8. Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007. 583:9–24.

9. Fries JW, Williams AJ, Atkins RC, Newman W, Liscomb MF, Collins T. Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am J Pathol. 1993. 143:725–737.
11. Nathan CF. Respiratory burst in adherent human neutrophils: Triggering by colony-stimulating factors CSF-GM and CSF-G. Blood. 1989. 73:301–306.
12. Eto M, Kozai T, Cosentino F, Joch H, Luscher TF. Statin prevents tissue factor expression in human endothelial cells: role of Rho/Rho-kinase and Akt pathways. Circulation. 2002. 105:1756–1759.
13. Inauen W, Granger DN, Meininger CJ, Schelling ME, Granger HJ, Kvietys PR. An in vitro model of ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1990. 259:G134–G139.

14. Yoshida N, Granger DN, Evans DJ Jr, Evans DG, Graham DY, Anderson DC, Wolf RE, Kvietys PR. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993. 105:1431–1440.

15. Son SM, Kim Ij, Kim YK. Study on role of neutrophil in endothelial cell injury under high glucose condition. J Korean Diabetes Assoc. 2000. 24:652–665.
16. Gannon DE, Varani J, Phan SH, Ward JH, Kaplan J, Till GO, Simon RH, Ryan US, Ward PA. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987. 57:37–44.
17. Ohara Y, Peterson TX, Sayegh HS, Subramanian RR, Wilcox JN, Harrison DG. Dietary correlation of hypercholesterolemia in the rabbit normalizes endothelial superoxide anion production. Circulation. 1995. 92:898–903.
18. Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993. 91:2546–2551.

20. Hashida S, Yuzawa S, Suzuki NN, Fujioka Y, Takikawa T, Sumimoto H, Inagaki F, Fujii H. Binding of FAD to cytochrome b558 is facilitated during activation of the phagocyte NADPH oxidase, leading to superoxide production. J Biol Chem. 2004. 279:26378–26386.

21. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000. 85:2970–2973.

22. Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr. 2002. 75:767–772.

23. Dandona P, Mohanty P, Hamouda W, Ghanim H, Aljada A, Garg R, Kumar V. Inhibitory effect of a two day fast on reactive oxygen species (ROS) generation by leucocytes and plasma ortho-tyrosine and meta-tyrosine concentrations. J Clin Endocrinol Metab. 2001. 86:2899–2902.
24. Hiramatsu K, Arimori S. Increased superoxide production by mononuclear cells of patients with hypertriglyceridemia and diabetes. Diabetes. 1988. 37:832–837.

25. Dandona P, Thusu K, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996. 347:444–445.

26. Hofmann MA, Schiekofer S, Kanitz M, Klevesath MS, Joswig M, Lee V, Morcos M, Tritschler H, Ziegler R, Wahl P, Bierhaus A, Nawroth PP. Insufficient glycemic control increases nuclear factor-kappa B binding activity in peripheral blood mononuclear cells isolated from patients with type 1 diabetes. Diabetes Care. 1998. 21:1310–1316.
27. Omori K, Ohira T, Uchida Y, Ayilavarapu S, Batista EL Jr, Yagi M, Iwata T, Liu H, Hasturk H, Kantarci A, Van Dyke TE. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol. 2008. 84:292–301.

28. Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam BH, Malabanan A, Trackman PC, Badwey JA, Van Dyke TE. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol. 2005. 78:862–870.

29. Beauchamp MC, Michaud SE, Li L, Sartippour MR, Renier G. Advanced glycation end products potentiate the stimulatory effect of glucose on macrophage lipoprotein lipase expression. J Lipid Res. 2004. 45:1749–1757.

30. A nderson, Heinecke JW. P roduction of N(epsilon)-(carboxymethyl)lysine is impaired in mice deficient in NADPH oxidase: a role for phagocyte-derived oxidants in the formation of advanced glycation end products during inflammation. Diabetes. 2003. 52:2137–2143.
31. McLeish KR, Knall C, Ward RA, Gerwins P, Coxon PY, Klein JB, Johnson GL. Activation of mitogen-activated protein kinase cascades during priming of human neutrophils by TNF-and GM-CSF. J Leukoc Biol. 1998. 64:537–545.
32. Perner A, Nielsen SE, Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Intensive Care Med. 2003. 29:642–645.

33. Curnutte JT, Badwey JA, Robinson JM, Karnovsky MJ, Karnovsky ML. Studies on the mechanism of superoxide release from human neutrophils stimulated with arachidonate. J Biol Chem. 1984. 259:11852–11857.

34. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000. 49:1939–1945.
35. Bannan S, Mansfield MW, Grant PJ. Soluble vascular cell adhesion molecule-1 and E-selectin levels in relation to vascular risk factors and to E-selectin genotype in the first degree relatives of NIDDM patients and in NIDDM patients. Diabetologia. 1998. 41:460–466.

36. Omi H, Okayama N, Shimizu M, Okouchi M, Ito S, Fukutomi T, Itoh M. Participation of high glucose concentrations in neutrophil adhesion and surface expression of adhesion molecules on cultured human endothelial cells: effect of antidiabetic medicines. J Diabetes Complications. 2002. 16:201–208.
37. Lee HS, Son SM, Kim YK, Hong KW, Kim CD. NAD(P)H oxidase participates in the signaling events in high glucose-induced proliferation of vascular smooth muscle cells. Life Sci. 2003. 72:2719–2730.

38. Kim BH, Lee CW, Park JL, Kang YH, Kim IJ, Kim YK, Son SM. High glucose modulates vascular smooth muscle cell proliferation through activation of PKC-δ-dependent NAD(P)H oxidase. J Korean Diabetes Assoc. 2006. 30:416–427.

39. Rask-Madsen C, King GL. P ro atherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005. 25:487–496.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download