Abstract
Background
Despite a recent breakthough in human islet transplantation for treating type 1 diabetes mellitus, the limited availability of donor pancreases remains a major obstacle. Endocrine cells within the gut epithelium (enteroendocrine cells) and pancreatic β cells share similar pathways of differentiation during embryonic development. In particular, K-cells that secrete glucose-dependent insulinotropic polypeptide (GIP) have been shown to express many of the key proteins found in β cells. Therefore, we hypothesize that K-cells can be transdifferentiated into β cells because both cells have remarkable similarities in their embryonic development and cellular phenotypes.
Methods
K-cells were purified from heterogeneous STC-1 cells originating from an endocrine tumor of a mouse intestine. In addition, a K-cell subclone expressing stable Nkx6.1, called "Kn4-cells," was successfully obtained. In vitro differentiation of K-cells or Kn4-cells into β cells was completed after exendin-4 treatment and serum deprivation. The expressions of insulin mRNA and protein were examined by RT-PCR and immunocytochemistry. The interacellular insulin content was also measured.
Results
K-cells were found to express glucokinase and GIP as assessed by RT-PCR and Western blot analysis. RT-PCR showed that K-cells also expressed Pdx-1, NeuroD1/Beta2, and MafA, but not Nkx6.1. After exendin-4 treatment and serum deprivation, insulin mRNA and insulin or C-peptide were clearly detected in Kn4-cells. The intracellular insulin content was also increased significantly in these cells.
Figures and Tables
Fig. 1
Design of Nkx6.1-expressing vector. The Nkx6.1-expressing vector (pcDNA3.1 + Nkx6.1) was made as shown in this cartoon.

Fig. 2
RT-PCR of transcription factors mRNA. K-cells were found to express transcription factors which were all present in mouse islets except Nkx6.1.
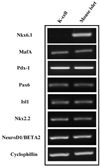
Fig. 3
RT-PCR of insulin 1 mRNA. Insulin1 band was weakly detected in K-cells after 7 day-treatment with 10 mM nicotinamide and 10 pM exendin-4 in serum-free medium. 1, Mouse islets; 1-1, K-cells; 2, K-cells, 7 days in serum-free medium; 3, K-cells, 7 days in serum-free medium with 10 mM nicotinamide; 4, K-cells, 7 days in serum-free medium with 10 mM nicotinamide and 10 pM exendin-4.
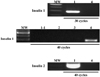
Fig. 4
RT-PCR of insulin 1 and Nkx6.1 mRNA. Insulin1 band was clearly detected in Kn4-cells after 7 day-treatment with 10 mM nicotinamide and 10 pM exendin-4 in serum-free medium. 1, Rat islets; 1-1, Mouse islets; 2, K-cells, 7 days in serum-free medium; 3, K_pcDNA-cells, 7 days in serum-free medium; 4, Kn4-cells, 7 days in serum-free medium; 5, K-cells, 7 days in serum-free medium with 10 mM nicotinamide and 10 pM exendin-4; 6, K_pcDNA-cells, 7 days in serum-free medium with 10 mM nicotinamide and 10 pM exendin-4; 7, Kn-4 cells, 7 days in serum-free medium with 10 mM nicotinamide and 10 pM exendin-4.
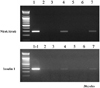
Fig. 5
Fluorescent microscopy for insulin immunocytochemistry in K-cells and transdifferentiated Kn4-cells. Some of differentiated Kn4-cells were found to be insulin-positive (A, Insulin staining in Ins-1 cells, ×200; B, Insulin staining in K-cells, ×200; C, Insulin staining in differentiated Kn4-cells, ×400; D, GFP expression, ×400).
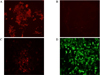
Fig. 6
Confocal microscopy for C-peptide immunocytochemistry in transdifferentiated Kn4-cells. Some of differentiated Kn4-cells were found to be C-peptide-positive (A, Nuclei staining with DAPI; B, GFP expression; C, C-peptide staining D, Merged image, ×400).
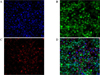
Fig. 7
Intracellular content of insulin. Insulin content was significantly higher in transdifferentiated Kn4-cells as
compared with K-cells. *P < 0.05.

References
1. Ko SH, Lee WY, Lee JH, Kwon HS, Lee JM, Kim SR, Moon SD, Song KH, Han JH, Ahn YB, Yoo SJ, Son HY. Clinical characteristics of diabetic ketoacidosis in Korea over the past two decades. Diabet Med. 2005. 22:466–469.
2. Ko SH, Song KH, Kim SR, Lee JM, Kim JS, Shin JH, Cho YK, Park YM, Jeong JH, Yoon KH, Cha BY, Son HY, Ahn YB. Long-term effects of a structured intensive diabetes education programme (SIDEP) in patients with Type 2 diabetes mellitus-a 4-year follow-up study. Diabet Med. 2007. 24:55–62.
3. You YH, Park SC, Lee SH, Park HS, Ham DS, Rhee M, Kim JW, Song KH, Yoon KH. PDX-1/VP16 Overexpression induce the transdifferentiation of canine adult pancreatic cells into beta-cells. J Korean Diabetes Assoc. 2007. 31:51–62.
4. Ko SH, Ryu GR, Kim S, Ahn YB, Yoon KH, Kaneto H, Ha H, Kim YS, Song KH. Inducible nitric oxide synthase-nitric oxide plays an important role in acute and severe hypoxic injury to pancreatic beta cells. Transplantation. 2008. 85:323–330.
5. Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007. 56:1802–1809.
6. Song KH, Kim MM, Lee MK, Ryu GR, Ko SH, Moon SD, Ahn YB, Yoon KH, Cha BY, Lee KW, Son HY, Kang SK, Chin HM. Differentiation of pancreatic beta cells from human pancreatic duct cells derived from a partial pancreas tissue. J Korean Diabetes Assoc. 2007. 31:236–242.
7. Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001. 292:1389–1394.
8. Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003. 21:763–770.
9. Song KH, Ko SH, Ahn YB, Yoo SJ, Chin HM, Kaneto H, Yoon KH, Cha BY, Lee KW, Son HY. In vitro transdifferentiation of adult pancreatic acinar cells into insulin-expressing cells. Biochem Biophys Res Commun. 2004. 316:1094–1100.
10. Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999. 400:877–881.
11. Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004. 229:176–200.
12. Vaca P, Martín F, Vegara-Meseguer JM, Rovira JM, Berná G, Soria B. Induction of differentiation of embryonic stem cells into insulin-secreting cells by fetal soluble factors. Stem Cells. 2006. 24:258–265.
13. Kritzik MR, Krahl T, Good A, Krakowski M, St-Onge L, Gruss P, Wright C, Sarvetnick N. Transcription factor expression during pancreatic islet regeneration. Mol Cell Endocrinol. 2000. 164:99–107.
14. Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005. 23:857–861.
15. Corbett JA. K cells: a novel target for insulin gene therapy for the prevention of diabetes. Trends Endocrinol Metab. 2001. 12:140–142.
16. Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe MM, Kieffer TJ. Glucose-dependent insulin release from genetically engineered K cells. Science. 2000. 290:1959–1962.
17. Ramshur EB, Rull TR, Wice BM. Novel insulin/GIP co-producing cell lines provide unexpected insights into Gut K-cell function in vivo. J Cell Physiol. 2002. 192:339–350.
18. Gagnon J, Mayne J, Mbikay M, Woulfe J, Chrétien M. Expression of PCSK1 (PC1/3), PCSK2 (PC2) and PCSK3 (furin) in mouse small intestine. Regul Pept. 2009. 152:54–60.
19. Kim JH, Moon SD, Ko SH, Ahn YB, Song KH, Lim HS, Lee SK, Yoo SJ, Son HS, Yoon KH, Cha BY, Son HY, Kim SJ, Han JH. Glucose-dependent insulin secretion from genetically engineered K-cells using EBV-based episomal vector. J Korean Diabetes Assoc. 2007. 31:9–21.
20. Jeon SY, Baek KH, Kim YS, Park CG, Kwon HS, Ko SH, Song KH, Yoo SJ, Son HS, Cha BY, Lee KW, Son HY, Kang SK, Yoon KH. Differentially up-regulated genes in proliferating porcine neonatal pancreas cells caused by epidermal growth factor. J Cell Biochem. 2004. 91:354–364.
21. Yasuda M, Yamamoto M, Ochiai H, Eguchi Y, Arishima K. Effect of growth factors on development of fetal islet B-cells in vitro. J Vet Med Sci. 2007. 69:807–811.
22. Mashima H, Ohnishi H, Wakabayashi K, Mine T, Miyagawa J, Hanafusa T, Seno M, Yamada H, Kojima I. Betacellulin and activin A coordinately convert amylase-secreting pancreatic AR42J cells into insulin-secreting cells. J Clin Invest. 1996. 97:1647–1654.
23. Yamaoka T, Idehara C, Yano M, Matsushita T, Yamada T, Ii S, Moritani M, Hata J, Sugino H, Noji S, Itakura M. Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. J Clin Invest. 1998. 102:294–301.
24. Mashima H, Yamada S, Tajima T, Seno M, Yamada H, Takeda J, Kojima I. Genes expressed during the differentiation of pancreatic AR42J cells into insulin-secreting cells. Diabetes. 1999. 48:304–309.
25. Mashima H, Shibata H, Mine T, Kojima I. Formation of insulin-producing cells from pancreatic acinar AR42J cells by hepatocyte growth factor. Endocrinology. 1996. 137:3969–3976.
26. Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci U S A. 2002. 99:16105–16110.
27. Cheon SJ, Kim JI, Lee JS. Effects of growth factors and kinase inhibitors on the properties of human adipose-stromal cells in different culture conditions. Cell Biol Int. 2008. 32:784–791.
28. Gao X, Song L, Shen K, Wang H, Niu W, Qin X. Transplantation of bone marrow derived cells promotes pancreatic islet repair in diabetic mice. Biochem Biophys Res Commun. 2008. 371:132–137.
29. Kodama S, Toyonaga T, Kondo T, Matsumoto K, Tsuruzoe K, Kawashima J, Goto H, Kume K, Kume S, Sakakida M, Araki E. Enhanced expression of PDX-1 and Ngn3 by exendin-4 during beta cell regeneration in STZ-treated mice. Biochem Biophys Res Commun. 2005. 327:1170–1178.
30. Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004. 117:77–88.
31. Movassat J, Beattie GM, Lopez AD, Hayek A. Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J Clin Endocrinol Metab. 2002. 87:4775–4781.
32. Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999. 48:2270–2276.
33. De León DD, Crutchlow MF, Ham JY, Stoffers DA. Role of glucagon-like peptide-1 in the pathogenesis and treatment of diabetes mellitus. Int J Biochem Cell Biol. 2006. 38:845–859.
34. Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci U S A. 2005. 102:15116–15121.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download