Abstract
Background
Streptozotocin-induced diabetic animals are characterized by hyperphagia due to deficiencies of insulin and leptin. Forkhead box-containing protein of the O subfamily-1 (FoxO1) regulates energy homeostasis by regulating energy expenditure and food intake as well as mediating insulin and leptin signals in the hypothalamus. To identify the mediator of diabetic hyperphagia, we examined the effects of insulin or leptin on hypothalamic FoxO1 expression in a diabetic animal model.
Methods
Diabetes was induced in mice (C57BL/6) by intraperitoneal administration of streptozotocin (200 mg/kg). Stainless steel cannula was implanted into the lateral ventricle of the brain in each mouse. After three weeks, the mice were administered saline, insulin or leptin via intracerebroventricular (ICV) route. The medial hypothalamus was isolated to evaluate the mRNA expressions of FoxO1 and neuropeptides.
Results
Streptozotocin-induced diabetic mice exhibited significant elevations of blood glucose and food intake and significantly low levels of serum insulin and leptin. The levels of hypothalamic FoxO1 mRNA were significantly increased in diabetic mice. The hypothalamic expression of neuropeptide Y (NPY) mRNA was increased, but the expression of preproopiomelanocortin (POMC) mRNA was decreased in diabetic mice. ICV administration of insulin or leptin attenuated the upregulation of hypothalamic FoxO1 mRNA, and resulted in downregulation of NPY mRNA and upregulation of POMC mRNA in diabetic mice.
Conclusion
We observed that the expression of hypothalamic FoxO1 mRNA was increased in streptozotocin-induced diabetic mice, and that it was significantly attenuated by central administration of insulin or leptin. These results suggest that hypothalamic FoxO1 is the direct mediator of diabetic hyperphagia.
Figures and Tables
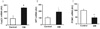 | Fig. 1Results of hypothalamic gene expression in streptozotocin induced diabetic mice. Levels of FoxO1, NPY and POMC gene expression in the hypothalamus of diabetic mice as determined by real time PCR. A, B, C. Expression levels of FoxO1, NPY and POMC mRNA were examined by real time PCR. Results are presented as means ± S.D. n = 5 per group. *P < 0.05, †P < 0.01 and ‡P < 0.001 vs. control mice. |
 | Fig. 2Effect of food deprivation on the mRNA level of hypothalamic FoxO1 and serum level of insulin and leptin. A, B, C. Expression levels of hypothalamic FoxO1, NPY and POMC mRNA in the animals of food deprivation for two days and in the animals of normally fed. D, E. Serum levels of insulin and leptin. Data are shown as means ± S.D. n = 5 per group; *P < 0.05, †P < 0.01 vs. control mice. |
 | Fig. 3Effect of i.c.v. administration of saline, insulin or leptin on hypothalamic FoxO1 expression in the streptozotocin induced diabetic mice. A, B, C. Expression levels of hypothalamic FoxO1, NPY, POMC mRNA in diabetic mice after i.c.v. administration of saline, insulin or leptin. Data are shown as the means ± S.D. n = 5 per group. *P < 0.05 and †P < 0.01 vs. control mice. ‡P < 0.05 and §P < 0.01 vs. diabetic mice. |
References
1. Booth DA. Some characteristics of feeding during streptozotocin-induced diabetes in the rat. J Comp Physiol Psychol. 1972. 80:238–249.
2. Hidaka S, Yoshimatsu H, Kondou S, Oka K, Tsuruta Y, Sakino H, Itateyama E, Noguchi H, Himeno K, Okamoto K, Teshima Y, Okeda T, Sakata T. Hypoleptinemia, but not hypoinsulinemia, induces hyperphagia in streptozotocin-induced diabetic rats. J Neurochem. 2001. 77:993–1000.
3. Namkoong C, Kim MS, Jang PG, Han SM, Park HS, Koh EH, Lee WJ, Kim JY, Park IS, Park JY, Lee KU. Enhanced hypothalamic AMP-activated protein kinase activity contributes to hyperphagia in diabetic rats. Diabetes. 2005. 54:63–68.
4. Havel PJ, Uriu-Hare JY, Liu T, Stanhope KL, Stern JS, Keen CL, Ahrén B. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998. 274:1482–1491.
5. Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995. 44:147–151.
6. Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW. Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes. 1999. 48:1275–1280.
7. Sindelar DK, Mystkowski P, Marsh DJ, Palmiter RD, Schwartz MW. Attenuation of diabetic hyperphagia in neuropeptide Y--deficient mice. Diabetes. 2002. 51:778–783.
8. Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006. 9:901–906.
9. Kitamura T, Feng Y, Kitamura YI, Chua SC Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006. 12:534–540.
10. Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008. 27:2320–2336.
11. Friedman JM. Modern science versus the stigma of obesity. Nat Med. 2004. 10:563–569.
12. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006. 443:289–295.
13. Williams G, Steel JH, Cardoso H, Ghatei MA, Lee YC, Gill JS, Burrin JM, Polak JM, Bloom SR. Increased hypothalamic neuropeptide Y concentrations in diabetic rat. Diabetes. 1988. 37:763–772.
14. Nakae J, Kitamura T, Kitamura Y, Biggs WH 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003. 4:119–129.
15. Buteau J, Accili D. Regulation of pancreatic beta-cell function by the forkhead protein FoxO1. Diabetes Obes Metab. 2007. 9:suppl 2. S140–S146.
16. Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008. 8:65–76.
17. Ropelle ER, Pauli JR, Prada P, Cintra DE, Rocha GZ, Moraes JC, Frederico MJ, da Luz G, Pinho RA, Carvalheira JB, Velloso LA, Saad MA, De Souza CT. Inhibition of hypothalamic Foxo1 expression reduced food intake in diet-induced obesity rats. J Physiol. 2009. 587:2341–2351.
18. Gelling RW. Diabetic hyperphagia--ghrelin in the driver's seat. Endocrinology. 2006. 147:2631–2633.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download