Abstract
Background
Skeletal muscle is the most important tissue contributing to insulin resistance. Several studies have shown that accumulation of intramyocellular lipid is associated with the development of insulin resistance. Thus, proteins involved in lipid transport, storage and metabolism might also be involved in insulin action in skeletal muscle. Adipose differentiation-related protein (ADRP), which is localized at the surface of lipid droplets, is known to be regulated by peroxisome proliferator activated receptor γ (PPARγ). However, it is not known whether ADRP plays a role in regulating glucose uptake and insulin action in skeletal muscle.
Methods
ADRP expression in skeletal muscle was measured by RT-PCR and western blot in db/db mice with and without PPARγ agonist. The effect of PPARγ agonist or high lipid concentration (0.4% intralipos) on ADRP expression was also obtained in cultured human skeletal muscle cells. Glucose uptake was measured when ADRP was down-regulated with siRNA or when ADRP was overexpressed with adenovirus.
Results
ADRP expression increased in the skeletal muscle of db/db mice in comparison with normal controls and tended to increase with the treatment of PPARγ agonist. In cultured human skeletal muscle cells, the treatment of PPARγ agonist or high lipid concentration increased ADRP expression. siADRP treatment decreased both basal and insulin-stimulated glucose uptake whereas ADRP overexpression increased glucose uptake in cultured human skeletal muscle cells.
Conclusion
ADRP expression in skeletal muscle is increased by PPARγ agonist or exposure to high lipid concentration. In these conditions, increased ADRP contributed to increase glucose uptake. These results suggest that insulin-sensitizing effects of PPARγ are at least partially achieved by the increase of ADRP expression, and ADRP has a protective effect against intramyocellular lipid-induced insulin resistance.
Figures and Tables
Fig. 1
Effect of rosiglitazone treatment on blood glucose, body weight and triglyceride (TG) in db/db mice. A. Blood glucose level. B. Body weight. C. Food intake. D. Water intake. E. Fasting plasma glucose. F. Fasting TG level at 14 days after rosiglitazone treatment. db/db + rosi: db/db + rosiglitazone treatment, *P < 0.01 vs. C57BL6J, †P < 0.01 vs. db/db mice.

Fig. 2
Effect of rosiglitazone on skeletal muscle ADRP expression in db/db mice. A. RT-PCR. B. Western blotting. db/db + rosi: db/db + rosiglitazone treatment. *P < 0.01 vs. C57BL6.

Fig. 3
Effect of PPARγ agonists on ADRP expression in cultured human skeletal muscle cells. Human skeletal muscle cells were differentiated and treated with 10 µM of troglitazone, rosiglitazone, pioglitazone, or wy14,643 for 48 h. *P < 0.01 vs. vehicle only.
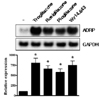
Fig. 4
Effect of ADRP knockdown on glucose uptake. Human skeletal muscle cells (HSkMC) were transfected with siRNAs of negative control (siNS) or ADRP (siADRP) for 12 h and treated with troglitazone for an additional 48 h. Prior to perform glucose uptake assay, cells were incubated with or without insulin (100 nM) for 30 min. (A) siADRP decreased both mRNA and protein expression of ADRP in HSkMC (B) glucose uptake in HSkMC. *P < 0.05: vs. basal value of control cells not treated with troglitazone; †P < 0.05: vs. corresponding value of control cells not treated with troglitazone, ‡P < 0.05 vs. corresponding value of cells treated with troglitazone and siNS.
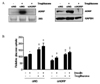
Fig. 5
Effect of ADRP overexpression on glucose uptake. A. Human skeletal muscle cells were transfected with adenovirus which expresses ADRP for 36 h. The increase in ADRP protein was determined by western blot. B. Glucose uptake in HSkMC. *P < 0.05 vs. the basal value of GFP adenovirus treated cells. †P < 0.05 vs. the corresponding value of GFP adenovirus transfected cells.
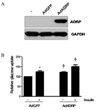
Fig. 6
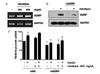
Role of ADRP on glucose uptake in cultured human skeletal muscle cells exposed to high lipid concentration A. High lipid (intralipos 400 mg/dL) induces ADRP mRNA expression in HSkMC. B. Human skeletal muscle cells were transfected with siNS or siADRP for 12 h and treated with high lipid for 48 h. siADRP inhibited high lipid-induced ADRP protein expression. C. Glucose uptake in siADRP transfected HSkMC with or without high lipid. *P < 0.05: vs. basal value of siNS treated cells. †P < 0.05: vs. corresponding value of siNS transfected cells not treated with high lipid, ‡P < 0.05: vs. corresponding value of cells treated with siNS and high lipid.
References
1. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004. 88:787–835.
2. Bouzakri K, Koistinen HA, Zierath JR. Molecular Mechanisms of Skeletal Muscle Insulin Resistance in Type 2 Diabetes. Curr Diabetes Rev. 20. 1:167–174.
3. Krssak M, FalkPetersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999. 42:113–116.
4. Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996. 45:947–950.
5. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997. 46:983–988.
6. Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, Kimmel AR. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome. 2001. 12:741–749.
7. Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997. 38:2249–2263.
8. Shaw CS, Sherlock M, Stewart PM, Wagenmakers AJ. Adipophilin distribution and colocalisation with lipid droplets in skeletal muscle. Histochem Cell Biol. 2009. 131:575–581.
9. Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998. 294:309–321.
10. Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008. 57:2037–2045.
11. Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res. 2007. 48:2751–2761.
12. Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005. 288:E1195–E1205.
13. Targett-Adams P, McElwee MJ, Ehrenborg E, Gustafsson MC, Palmer CN, McLauchlan J. A PPAR response element regulates transcription of the gene for human adipose differentiation-related protein. Biochim Biophys Acta. 2005. 1728:95–104.
14. Motomura W, Inoue M, Ohtake T, Takahashi N, Nagamine M, Tanno S, Kohgo Y, Okumura T. Up-regulation of ADRP in fatty liver in human and liver steatosis in mice fed with high fat diet. Biochem Biophys Res Commun. 2006. 340:1111–1118.
15. Mak KM, Ren C, Ponomarenko A, Cao Q, Lieber CS. Adipose differentiation-related protein is a reliable lipid droplet marker in alcoholic fatty liver of rats. Alcohol Clin Exp Res. 2008. 32:683–689.
16. Edvardsson U, Ljungberg A, Linden D, William-Olsson L, Peilot-Sjogren H, Ahnmark A, Oscarsson J. PPARalpha activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes. J Lipid Res. 2006. 47:329–340.
17. Dalen KT, Ulven SM, Arntsen BM, Solaas K, Nebb HI. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J Lipid Res. 2006. 47:931–943.
18. Chawla A, Lee CH, Barak Y, He W, Rosenfeld J, Liao D, Han J, Kang H, Evans RM. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci U S A. 2003. 100:1268–1273.
19. Jun Gao, Hong Ye, Ginette Serrero. Stimulation of adipose differentiation related protein (ADRP) expression in adipocyte precursors by long-chain fatty acids. J Cell Physiol. 2000. 182:297–302.
20. Phillips SA, Choe CC, Ciaraldi TP, Greenberg AS, Kong AP, Baxi SC, Christiansen L, Mudaliar SR, Henry RR. Adipocyte differentiation-related protein in human skeletal muscle: relationship to insulin sensitivity. Obes Res. 2005. 13:1321–1329.
21. Magnusson Björn, Asp Lennart, Boström Pontus, Ruiz Michel, Stillemark-Billton Pia, Lindén Daniel, Borén Jan, Olofsson Sven-Olof. Adipocyte Differentiation-Related Protein Promotes Fatty Acid Storage in Cytosolic Triglycerides and Inhibits Secretion of Very Low-Density Lipoproteins. Arterioscler Thromb Vasc Biol. 2006. 26:1566–1571.
22. Jiang HP, Serrero G. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proc Natl Acad Sci USA. 1992. 89:7856–7860.
23. Jenkins AB, Storlien LH, Chisholm DJ, Kraegen EW. Effects of nonesterified fatty acid availability on tissue-specific glucose utilization in rats in vivo. J Clin Invest. 1988. 82:293–299.
24. Hirabara SM, Silveira LR, Abdulkader F, Carvalho CR, Procopio J, Curi R. Time-dependent effects of fatty acids on skeletal muscle metabolism. J Cell Physiol. 2007. 210:7–15.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download