Abstract
Background
Vascular endothelial growth factor (VEGF) is associated with the development of diabetic complications. However, it is unknown whether systemic VEGF treatment has any effects on the pancreatic islets in an animal model of type 2 diabetes mellitus.
Methods
Anti-VEGF peptide (synthetic ATWLPPR, VEGF receptor type 2 antagonist) was injected into db/db mice for 12 weeks. We analyzed pancreatic islet morphology and quantified beta-cell mass. Endothelial cell proliferation and the severity of islet fibrosis were also measured. VEGF expression in isolated islets was determined using Western blot analysis.
Results
When anti-VEGF was administered, db/db mice exhibited more severe hyperglycemia and associated delayed weight gain than non-treated db/db mice. Pancreas weight and pancreatic beta-cell mass were also significantly decreased in the anti-VEGF-treated group. VEGF and VEGF receptor proteins (types 1 and 2) were expressed in the pancreatic islets, and their expression was significantly increased in the db/db group compared with the db/dm group. However, the elevated VEGF expression was significantly reduced by anti-VEGF treatment compared with the db/db group. The anti-VEGF-treated group had more prominent islet fibrosis and islet destruction than db/db mice. Intra-islet endothelial cell proliferation was also remarkably reduced by the anti-VEGF peptide.
Conclusion
Inhibition of VEGF action by the VEGF receptor 2 antagonist not only suppressed the proliferation of intra-islet endothelial cells but also accelerated pancreatic islet destruction and aggravated hyperglycemia in a type 2 diabetes mouse model. Therefore, the potential effects of anti-VEGF treatment on pancreatic beta cell damage should be considered.
Figures and Tables
 | Fig. 1Changes in body weight. Compared with the db/db group, the anti-VEGF-treated group had a significantly lower body weight after 3 weeks of anti-VEGF injections. |
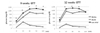 | Fig. 2The result of intraperitoneal glucose tolerance tests (IPGTTs). Glucose concentrations during IPGTTs conducted 9 and 12 weeks after the start of the trial showed remarkable differences between the anti-VEGF and diabetic control groups. The anti-VEGF group had greater hyperglycemia than that of the db/db group (*P < 0.05). |
 | Fig. 3The area under the curve (AUCg) of the intraperitoneal glucose tolerance test. Compared with the nondiabetic db/dm control group, the diabetic db/db and anti-VEGF-treated mice had significantly elevated AUCg values. Values are means (n = 10 per group). *P < 0.05 vs. nondiabetic control (db/dm) and diabetic control (db/db) mice. |
 | Fig. 4Mean pancreas masses (left) and beta-cell masses (right) for the experimental groups. Compared with the db/db and db/dm groups, the anti-VEGF-treated group had decreased pancreas and beta-cell masses. Values are means (n = 10 per group). *P < 0.05 vs. nondiabetic controls, †P < 0.05 vs. diabetic control db/db mice. |
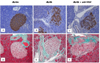 | Fig. 5Immunohistochemical analysis of pancreatic islets using insulin (A-C) and trichrome (D-F) staining. Sections of paraffin-embedded islets from db/dm (A), db/db (B) and anti-VEGF-treated (C) mice were immunostained with anti-insulin antibody (brown color). Compared with the nondiabetic control group, the pancreatic islet architecture of the db/db (B) and anti-VEGF-treated (C) mice was severely disorganized and the area that stained positively for insulin was decreased (×200). The lower panel shows islet fibrosis as a blue stain. Compared with the nondiabetic control group (D), islet fibrosis was remarkably increased in both the db/db (E) and anti-VEGF-treated groups (F), which also showed islet destruction. Magnification is 200× that of the original. |
 | Fig. 6Immunohistochemical analysis of pancreatic islets using VEGF staining. Islets were stained with anti-VEGF antibody. VEGF protein (brown color) was expressed mainly in the periphery of the pancreatic islets. VEGF expression in anti-VEGF-treated tissue was significantly decreased. Magnification is 200× that of the original. |
 | Fig. 7Relative percentage VEGF expression and area of fibrosis. In anti-VEGF-treated mice, the relative percentage VEGF expression in the islets was decreased and the area of islet fibrosis was remarkably increased compared with the other two groups. *P < 0.05 vs. nondiabetic controls; †P < 0.05 vs. diabetic control db/db mice. Values are means (%). |
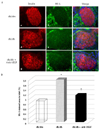 | Fig. 8(A) Visualization of vascular endothelial cells in the islets of db/dm (a-c), db/db (d-e) and anti-VEGF-treated (g-i) mice using immunohistochemical staining for BS-1 in islets. Co-staining for insulin (red), BS-1 (green) and DAPI (blue) are shown. Merged images are shown in c, f and i. BS-1 stained cells in the islets are evident as bright green dots (b, e, h). BS-1 expression was remarkably decreased in the anti-VEGF-treated group (h). Magnification is 200× that of the original. (B) Quantification of BS-1-positive cell numbers in islets of individual groups. Each bar represents counts from 10 to 15 sections from 10 mice per group. *P < 0.05 vs. nondiabetic controls; †P < 0.05 vs. diabetic control db/db mice. Values are means. |
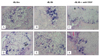 | Fig. 9In situ hybridization analysis of VEGF-R1 (A-C) and VEGF-R2 (D-F) expression in pancreatic islets. Both VEGF receptors were expressed in the pancreatic islets of all experimental groups, and at a lower level in exocrine tissue. In addition to VEGF R1, VEGF R2 expression was also decreased in the anti-VEGF-treated group (C, F). The magnification in A-F is 200×. |
 | Fig. 10Expression of VEGF in isolated islets from db/dm, db/db, and anti-VEGF-treated mice (A). VEGF expression was remarkably increased in the islets of db/db mice (*P < 0.05 vs. nondiabetic controls), but was decreased in the islets of VEGF-treated mice (†P < 0.05 vs. diabetic control db/db mice) (B). Values are means. |
References
1. Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002. 51:3090–3094.
2. de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001. 12:993–1000.
3. Simorre-Pinatel V, Guerrin M, Chollet P, Penary M, Clamens S, Malecaze F, Plouet J. Vasculotropin-VEGF stimulates retinal capillary endothelial cells through an autocrine pathway. Invest Ophthalmol Vis Sci. 1994. 35:3393–3400.
4. Vasir B, Jonas JC, Steil GM, Hollister-Lock J, Hasenkamp W, Sharma A, Bonner-Weir S, Weir GC. Gene expression of VEGF and its receptors Flk-1/KDR and Flt-1 in cultured and transplanted rat islets. Transplantation. 2001. 71:924–935.
5. Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, Zhang H, Song K, Meseck M, Bromberg J, Dong H. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004. 53:963–970.
6. Stagner J, Mokshagundam S, Wyler K, Samols E, Rilo H, Stagner M, Parthasarathy L, Parthasarathy R. Beta-cell sparing in transplanted islets by vascular endothelial growth factor. Transplant Proc. 2004. 36:1178–1180.
7. Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med. 2005. 9:777–794.
8. Konstantinova I, Lammert E. Microvascular development: learning from pancreatic islets. Bioessays. 2004. 26:1069–1075.
9. Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006. 290:H560–H576.
10. Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005. 65:550–563.
11. Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2000. 13:1070–1074.
12. Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006. 10:397–405.
13. Christofori G, Naik P, Hanahan D. Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol Endocrinol. 1995. 9:1760–1770.
14. Binétruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, Plouët J, Derbin C, Perret G, Mazié JC. Identification of a peptide blocking vascular endothelial growth Factor (VEGF)-mediated angiogenesis. EMBO J. 2000. 19:1525–1533.
15. Zilberberg L, Shinkaruk S, Lequin O, Rousseau B, Hagedorn M, Costa F, Caronzolo D, Balke M, Canron X, Convert O, Laïn G, Gionnet K, Goncalvès M, Bayle M, Bello L, Chassaing G, Deleris G, Bikfalvi A. Structure and inhibitory effects on angiogenesis and tumor development of a new vascular endothelial growth inhibitor. J Biol Chem. 2003. 278:35564–35573.
16. Weibel ER. Stereologic methods. Practical Methods for Biologic Morphometry. 1978. Vol. 1. London: Academic Press;101–161.
17. Mattsson G. The endothelial cells in islets of langerhans. Ups J Med Sci. 2005. 110:1–15.
18. Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967. 16:35–39.
19. Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, O'Hara CJ, Monaco AP. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987. 43:725–730.
20. Iwashita N, Uchida T, Choi JB, Azuma K, Ogihara T, Ferrara N, Gerber H, Kawamori R, Inoue M, Watada H. Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic beta cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia. 2007. 50:380–389.
21. Xie K, Wei D, Shi Q, Huang S. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev. 2004. 15:297–324.
22. Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003. 28:488–494.
23. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997. 18:4–25.
24. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999. 13:9–22.
25. Li X, Zhang L, Meshinchi S, Dias-Leme C, Raffin D, Johnson JD, Treutelaar MK, Burant CF. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes. 2006. 55:2965–2973.
26. Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006. 147:2315–2324.
27. Kakizawa H, Itoh M, Itoh Y, Imamura S, Ishiwata Y, Matsumoto T, Yamamoto K, Kato T, Ono Y, Nagata M, Hayakawa N, Suzuki A, Goto Y, Oda N. The relationship between glycemic control and plasma vascular endothelial growth factor and endothelin-1 concentration in diabetic patients. Metabolism. 2004. 53:550–555.
28. Linn T, Schneider K, Hammes HP, Preissner KT, Brandhorst H, Morgenstern E, Kiefer F, Bretzel RG. Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J. 2003. 17:881–883.
29. Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998. 47:401–406.
30. Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, Bucala R, Adamis AP. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest. 1998. 1:1219–1224.
31. Kuroki M, Voest EE, Amano S, Beerepoot LV, Takashima S, Tolentino M, Kim RY, Rohan RM, Colby KA, Yeo KT, Adamis AP. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996. 98:1667–1675.
32. Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC-b inhibitor. Science. 1996. 272:728–731.
33. Pieramici DJ, Rabena MD. Anti-VEGF therapy: comparison of current and future agents. Eye. 2008. 22:1330–1336.
34. Nagpal M, Nagpal K, Nagpal PN. A comparative debate on the various anti-vascular endothelial growth factor drugs: pegaptanib sodium (Macugen), ranibizumab (Lucentis) and bevacizumab (Avastin). Indian J Ophthalmol. 2007. 55:437–439.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download