Abstract
Oxidative stress has been considered to be a major contributor to the pathogenesis of the diabetic macrovascular and microvascular complications. In the absence of an appropriate antioxidant defense mechanism, increased oxidative stress leads to the activation of stress-sensitive intracellular signaling pathways and the formation of gene products that cause damage and contribute to the late complications of diabetes. The source of reactive oxygen species (ROS) in the pancreatic beta cells and insulin sensitive cells has postulated to be the mitochondrial electron transport chain. NAD(P)H oxidase-dependent ROS production is also important as the source both in pancreatic beta cells and other cells. NAD(P)H oxidase mediated ROS can alter parameters of signal transduction, insulin secretion, insulin action, cell proliferation and cell death. Additionally, oxidative stress as the pathogenic mechanism linking insulin resistance with dysfunction of both pancreatic beta cells and endothelial cells, eventually leads to diabetes and its complications. Further investigation of the mechanisms and its therapeutic interventions based on focusing NAD(P)H oxidase associated ROS production in the islet cells and other islet cells are needed.
Figures and Tables
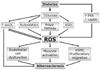 | Fig. 1Overview of the sources of ROS in diabetes and their links to atherosclerosis. oxLDL, oxidized LDL; FFA, free fatty acid; AGEs, advanced glycation end-products; VSMC, vascular smooth muscle cells; ROS, reactive oxygen species. |
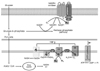 | Fig. 2Production of reactive oxygen species (ROS) in a generic cell type (Adapted from Anteriosclero Thromb Vasc Biol 24:816-23, 2004 & J Physiol 583:9-24, 2007). |
References
1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the developmentand progression of long-term complication in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.
2. UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998. 352:837–853.
3. Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000. 87:1123–1132.
4. Hunt JV, Dean RT, Wolff SP. Hydroxy radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988. 256:205–212.
5. Schmidt AM, Hori O, Brett J, Yan SD, Wautler JL, Stern D. Cellular receptors for advanced glycation end products: implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscle Thromb Vasc Biol. 1994. 14:1521–1528.
6. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002. 23:599–622.
7. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003. 552:335–344.
8. Fridlyand LE, Philipson LH. Oxidative reactive species in cell injury: Mechanisms in diabetes mellitus and therapeutic approaches. Ann N Y Acad Sci. 2005. 1066:136–151.
9. Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H. Increased glucose uptake promotes oxidative stress and PKC-δ activation in adipocytes of obese, insulin resistant mice. Am J Physiol Endocrinol Metab. 2003. 285:E295–E302.
10. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005. 54:1615–1625.
11. Haber EP, Procopio J, Carvalho CR, Carpinelli AR, Newsholme P, Curi R. New insights into fatty acid modulation of pancreatic β-cell function. Int Rev Cytol. 2006. 248:1–41.
12. Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003. 108:2034–2040.
13. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000. 49:1939–1945.
14. Son SM, Whalin MK, Harrison DG, Griendling KK. Oxidative Stress and diabetic vascular complications. Curr Diab Rep. 2004. 4:247–252.
15. Kiuchi K, Nejima J, Takano T, Ohta M, Hashimoto M. Increased serum concentrations of advanced glycation end products: a marker of coronary artery disease activity in type 2 diabetic patients. Heart. 2001. 85:87–91.
16. Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998. 4:1025–1030.
17. Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Srivastava SK. Nitric oxide regulates the polyol pathway of glucose metabolism in vascular smooth muscle cells. FASEB J. 2003. 17:417–425.
18. Wauters M, Considine RV, Yudkin JS, Peiffer F, De Leeuw I, Van Gaal LF. Leptin levels in type 2 diabetes: associations with measures of insulin resistance and insulin secretion. Horm Metab Res. 2003. 35:92–96.
19. Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999. 13:1231–1238.
20. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963. 1:785–789.
21. Garlid KD, Orosz DE, Modriansky M, Vassanelli S, Jezek P. On the mechanism of fatty acid-induced protein transport by mitochondrial uncoupling protein. J Biol Chem. 1996. 271:2615–2620.
22. Jaburek M, Varecha M, Gimeno RE, Dembski M, Jezek P, Zhang M, Burn P, Tartaglia LA, Garlid KD. Transport function and regulation of mitochondrial uncoupling proteins 2 and 3. J Biol Chem. 1999. 274:26003–26007.
23. Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997. 416:15–18.
24. Babior BM. NADPH oxidase. Curr Opin Immunol. 2004. 16:42–47.
25. Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology. 2006. 21:269–280.
26. Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005. 25:487–496.
27. Nakayama M, Inoguchi T, Sonta T, Maeda Y, Sasaki S, Sawada F, Tsubouchi H, Sonoda N, Kobayashi K, Sumimoto H, Nawata H. Increased expression of NAD(P)H oxidase in islets of animal models of Type 2 diabetes and its improvement by an AT1 receptor antagonist. Biochem Biophys Res Commun. 2005. 332:927–933.
28. Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic β-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003. 52:1457–1463.
29. Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, Curi R, Newsholme P, Carpinelli AR. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal β cell line. Diabetologia. 2007. 50:359–369.
30. Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007. 406:105–114.
31. Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000. 87:179–183.
32. Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996. 271:15703–15707.
33. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007. 39:44–84.
34. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007. 87:245–313.
35. Schoonbroodt S, Piette J. Oxidative stress interference with the nuclear factor-κ B activation pathways. Biochem Pharmacol. 2000. 60:1075–1083.
36. Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol. 2005. 25:1142–1147.
37. Newsholme P, Brennan L, Bender K. Amino acid metabolism, β-cell function, and diabetes. Diabetes. 2006. 55:Suppl 2. S39–S47.
38. Newsholme P, Keane D, Welters HJ, Morgan NG. Life and death decisions of the pancreatic β-cell: the role of fatty acids. Clin Sci (Lond). 2007. 112:27–42.
39. Kahn SE. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003. 46:3–19.
40. Yu JH, Kim KH, Kim H. Role of NADPH oxidase and calcium in cerulein-induced apoptosis: involvement of apoptosis-inducing factor. Ann N Y Acad Sci. 2006. 1090:292–297.
41. Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007. 583:9–24.
42. McQuaid TS, Saleh MC, Joseph JW, Gyulkhandanyan A, Manning-Fox JE, MacLellan JD, Wheeler MB, Chan CB. cAMP-mediated signaling normalizes glucose-stimulated insulin secretion in uncoupling protein-2 overexpressing β-cells. J Endocrinol. 2006. 190:669–680.
43. Chan CB, Kashemsant N. Regulation of insulin secretion by uncoupling protein. Biochem Soc Trans. 2006. 34:802–805.
44. Saleh MC, Wheeler MB, Chan CB. Endogenous islet uncoupling protein-2 expression and loss of glucose homeostasis in ob/ob mice. J Endocrinol. 2006. 190:659–667.
45. Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA, Matsuhisa M, Yamasaki Y. Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic β-cell dysfunction and insulin resistance. Int J Biochem Cell Biol. 2006. 38:782–793.
46. Paolisso G, Giuglianoc D. Oxidative stress and insulin action: Is there a relationship? Diabetologia. 1996. 39:357–363.
47. Cheatham B, Kahn CR. Insulin action and the insulin signaling network. Endocr Rev. 1995. 16:117–142.
48. Chen R, Kim O, Yang J. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001. 276:31858–31862.
49. Sykiotis GP, Papavassiliou AG. Serine phosphorylation of insulin receptor substrate-1; a novel target for the reversal of insulin resistance. Mol Endocrinol. 2001. 15:1864–1869.
50. Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by serine 312 phosphorylation. J Biol Chem. 2003. 278:8199–8211.
51. Hirata AE, Alvarez-Rojas F, Carvalheira JB, Carvalho CR, Dolnikoff MS, Abdalla Saad MJ. Modulation of IR/PTP1B interaction and downstream signaling in insulin sensitive tissue of MSG-rats. Life Sci. 2003. 73:1369–1381.
52. De Fea K, Roth RA. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry. 1997. 36:12939–12947.
53. Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002. 277:1531–1537.
54. Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001. 50:24–31.
55. Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005. 87:99–109.
56. Bloch-Damti A, Potashnik R, Gual P, Le Marchand-Brustel Y, Tanti JF, Rudich A, Bashan N. Differential effects of IRS1 phosphorylated on Ser307 or Ser632 in the induction of insulin resistance by oxidative stress. Diabetologia. 2006. 49:2463–2473.
57. Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem. 2002. 277:30010–30018.
58. Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002. 420:333–336.
59. Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka TA, Kajimoto Y, Matsuhisa M, Yamasaki Y, Hori M. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem. 2004. 279:45803–45809.
60. Dey D, Mukherjee M, Basu D, Datta M, Roy SS, Bandyopadhyay A, Bhattacharya S. Inhibition of insulin receptor gene expression and insulin signaling by fatty acid: interplay of PKC isoforms therein. Cell Physiol Biochem. 2005. 16:217–228.
61. Greene MW, Ruhoff MS, Roth RA, Kim JA, Quon MJ, Krause JA. PKCδ-mediated IRS-1 Ser24 phosphorylation negatively regulates IRS-1 function. Biochem Biophys Res Commun. 2006. 349:976–986.
62. Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor κ B kinase complex. J Biol Chem. 2002. 277:48115–48121.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download