Abstract
Background
Oxidative stress contributes to vascular diseases in patients with diabetes. As the mechanism of development and progression of diabetic vascular complications is poorly understood, this study was aimed to assess the potential role of hyperglycemia-induced oxidative stress and to determine whether the oxidative stress is a major factor in hyperglycemia-induced migration of vascular smooth muscle cells (VSMCs).
Methods
We treated primary cultured rat aortic smooth muscle cells for 72 hours with medium containing 5.5 mM D-glucose (normal glucose), 30 mM D-glucose (high glucose) or 5.5 mM D-glucose plus 24.5 mM mannitol (osmotic control). We measured the migration of VSMCs and superoxide production. Immunoblotting of PKC isozymes using phoshospecific antibodies was performed, and PKC activity was also measured.
Results
Migration of VSMCs incubated under high glucose condition were markedly increased compared to normal glucose condition. Treatment with diphenyleneiodonium (DPI, 10 µmol/L) and superoxide dismutase (SOD, 500 U/mL) significantly suppressed high glucose-induced migration of VSMCs. Superoxide production was significantly increased in high glucose condition and was markedly decreased after treatment with DPI and SOD. High glucose also markedly increased activity of PKC-δ isozyme. When VSMCs were treated with rottlerin or transfected with PKC-δ siRNA, nitro blue tetrazolium (NBT) staining and NAD(P)H oxidase activity were significantly attenuated in the high glucose-treated VSMCs. Furthermore, inhibition of PKC-δ markedly decreased VSMC migration by high glucose.
Figures and Tables
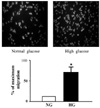 | Fig. 2Effect of high glucose on the migration of VSMCs. Data are shown as the mean ± SE from 4 experiments in each group. *P < 0.01 vs. normal glucose. NG, normal glucose; HG, high glucose. |
 | Fig. 3Effect of high glucose on the ROS production in vascular smooth muscle cell. Dihydroethidium (DHE) staining for superoxide anion (×100). |
 | Fig. 4Effect of various inhibitors such as DPI, NAC, SOD, L-NAME, and allopurinol on the superoxide generation in normal and high glucose-treated vascular smooth muscle cells (VSMCs). Data are shown as mean ± SE from 4 experiments in each group. *P < 0.05 vs. normal glucose. †P < 0.01 compared with corresponding values in each vehicle. |
 | Fig. 5Effects of various inhibitors, such as such as DPI (10 µmol/L), SOD (500 U/mL), allopurinol (Allo, 100 µmol/L), rotenone (Rot, 100 µmol/L), L-NAME (NMA, 10 µmol/L), and indomethacin (IND, 10 µmol/L), NADH-stimulated superoxide production in normal and high glucose-treated VSMCs. Data are shown as mean ± SE from 4 experiments in each group. *P < 0.01 vs. normal glucose. †P < 0.01 vs. vehicle (veh). |
 | Fig. 6Effects of high glucose on in vitro PKC activity in VSMCs. Results were expressed as a mean percentage of 5.5 mmol/L D-glucose ± SE from 4 independent experiments. *P < 0.01 vs. normal glucose (5.5 mM). |
 | Fig. 7Effects of high glucose on in vitro PKC activity and isozyme selective inhibition of PKC-δ in VSMCs. A. Confluent cultured VSMCs were treated with high glucose condition for 72 h. To verify the efficacy and selectivity of isozyme specific PKC inhibitor, VSMCs were treated with rottlerin (3 µmol/L) for 1 h before incubation with high glucose for 72 h, lysed and immunoblotted by using PKC isozyme-specific antibodies recognizing phosphorylated forms of PKC-δ. B. Quiescent VSMCs were transfected with PKC-δ siRNA prior to stimulation with high glucose for 72 h. Total PKC isozymes in the immunoprecipitates were verified by immunoblotting with selective antibodies. The bar graph represents the mean ± SE of three separate experiments. *P < 0.01 compared with normal glucose. †P < 0.01 compared with high glucose without pretreatment with rottlerin. |
 | Fig. 8Effect of transient transfections of PKC-δ siRNA on cellular superoxide production in normal and high glucose-treated VSMCs. Superoxide production was measured by NBT reduction. Results are presented as means ± SE for three independent experiments. *P < 0.05 vs. normal glucose. †P < 0.01 vs. vehicle. |
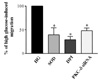 | Fig. 9Effect of DPI, selective PKC inhibitor or PKC-δ siRNA on VSMC migration.Quiescent VSMCs were pretreated with DPI (10 µmol/L), rottlerin (3 µmol/L) for 1 h or transfected with PKC-δ siRNA prior to stimulation with high glucose. Results are obtained from three separate experiments and are expressed in the mean ± SE. *P < 0.05 compared with high glucose without treatment. |
References
1. Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974. 23:105–111.

2. Ross R. The pathogenesis of atherosclerosisan update. N. Engl. J. Med. 1986. 314:488–500.
3. Natarajan R, Gonzales N, Xu L, Nadler J. Vascular smooth muscle cells exhibit increased growth response to elevated glucose. Biochem Biophys Res Commun. 1992. 87:552–560.
4. Hayashi JN, Ito H, Kanayasu T, Asuwa N, Morita I, Ishii T, Murota S. Effects of glucose on migration, proliferation and tube formation by vascular endothelial cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991. 60:245–252.

5. Yasunari K, Kohno M, Kano H, Yokokawa K, Minami M, Yoshikawa J. Mechanisms of action of troglitazone in the prevention of high glucose induced migration and proliferation of cultured coronary smooth muscle cells. Circ Res. 1997. 81:953–962.
6. Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003. 108:2034–2040.
7. Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001. 88:E14–E22.

8. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002. 23:599–622.

9. Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood). 2006. 231:237–251.

10. Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000. 28:1815–1826.

12. Yasunari K, Kohno M, Kano H, Yokokawa K, Minami M, Yoshikawa J. Antioxidants improve impaired insulin-mediated glucose uptake and prevent migration and proliferation of cultured rabbit coronary smooth muscle cells induced by high glucose. Circulation. 1999. 99:1370–1378.

13. Park JY, Park KG, Kim HJ, Kang HG, Ahn JD, Kim HS, Kim YM, Son SM, Kim IJ, Kim YK, Kim CD, Lee KU, Lee IK. The effects of the overexpression of recombinant uncoupling protein 2 on proliferation, migration and plasminogen activator inhibitor 1 expression in human vascular smooth muscle cells. Diabetologia. 2005. 48:1022–1028.

14. Lee HS, Son SM, Kim YK, Hong KW, Kim CD. NAD(P)H oxidase participates in the signaling events in high glucose-induced proliferation of vascular smooth muscle cells. Life Sci. 2003. 72:2719–2730.

15. Schwartz SM. Perspectives series: cell adhesion in vascular biology. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997. 99:2814–2816.

16. Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992. 89:507–511.

17. Jackson CL, Raines EW, Ross R, Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993. 13:1218–1226.

18. Bell L, Madri JA. Influence of the angiotensin system on endothelial and smooth muscle cell migration. Am J Pathol. 1990. 137:7–12.
19. Bornfeldt KE, Raines EW, Nakano T, Graves LM, Krebs EG, Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994. 93:1266–1274.

20. Nelson PR, Yamamura S, Kent KC. Extracellular matrix proteins are potent agonists of human smooth muscle cell migration. J Vasc Surg. 1996. 24:25–32.

21. Bell L, Madri JA. Effect of platelet factors on migration of cultured bovine aortic endothelial and smooth muscle cells. Circ Res. 1989. 65:1057–1065.

22. Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007. 100:607–621.

23. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000. 49:1939–1945.

24. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase; role in cardiovascular biology and disease. Circ Res. 2000. 86:494–501.
25. Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy. PartI. Circulation. 2003. 108:1527–1532.
26. Nishio E, Watanabe Y. The involvement of reactive oxygen species and arachidonic acid in alpha 1-adrenoceptor-induced smooth muscle cell proliferation and migration. Br J Pharmacol. 1997. 121:665–670.
27. Wang Z, Castresana MR, Newman WH. Reactive oxygen and NF-kappaB in VEGF-induced migration of human vascular smooth muscle cells. Biochem Biophys Res Commun. 2001. 285:669–674.
28. Wang Z, Castresana MR, Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004. 36:49–56.

29. Taniyama Y, Weber DS, Rocic P, Hilenski L, Akers ML, Park J, Hemmings BA, Alexander RW, Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol. 2003. 23:8019–8029.

30. Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001. 21:489–495.

31. Eguchi S, Iwasaki H, Inagami T, Numaguchi K, Yamakawa T, Motley ED, Owada KM, Marumo F, Hirata Y. Involvement of PYK2 in angiotensin II signaling of vascular smooth muscle cells. Hypertension. 1999. 33:201–206.

32. Leduc I, Meloche S. Angiotensin II stimulates tyrosine phosphorylation of the focal adhesion associated protein paxillin in aortic smooth muscle cells. J Biol Chem. 1995. 270:4401–4404.
33. Okuda M, Kawahara Y, Nakayama I, Hoshijima M, Yokoyama M. Angiotensin II transduces its signal to focal adhesions via angiotensin II type 1 receptors in vascular smooth muscle cells. FEBS Lett. 1995. 368:343–347.

34. Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda). 2006. 21:269–280.

35. King GL, Kunisaki M, Nishi N, Inoguchi T, Shiba T, Xia P. Biochemical and and molecular mechanisms in the development of diabetic vascular complications. Diabetes. 1996. 45:Suppl 3. S105–S108.
36. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000. 49:1939–1944.

37. Li C, Wernig F, Leitges M, Hu Y, Xu Q. Mechanical stress-activated PKC delta regulates smooth muscle cell migration. FASEB J. 2003. 17:2106–2108.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download