Abstract
Background
Insulin resistance is very common in patients with nonalcoholic fatty liver disease (NAFLD). Glitazones improve insulin sensitivity by acting as a selective agonist of the peroxisome proliferators-activated receptor gamma (PPAR γ), and were shown to activate AMP-activated protein kinase (AMPK) in skeletal muscle and the liver. Glitazones were also shown to reduce hepatic lipogenesis. The aim of this study was to investigate whether the protective mechanism of rosiglitazone on NAFLD is associated with AMPK activation.
Methods
Twelve OLETF rats were divided into 2 groups (control, treatment, n = 6 each). LETO rats served as controls. At 35 weeks of age, treatment group received rosiglitazone 4 mg/kg daily for 3 days. Fasting plasma glucose, insulin, free fatty acid, lactate and triglycerides were measured. Liver tissues from each group were processed for histological and hepatic triglyceride content analysis and western blotting.
Results
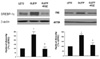
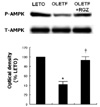
Fasting plasma glucose, insulin and triglycerides levels were significantly lower in treatment group than in control group. Histologic examination disclosed decreased hepatic steatosis in treatment group. Hepatic triglyceride content was also decreased in treatment group. Sterol regulatory binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) expression were increased and AMPK phosphorylation was reduced in OLETF rats compared with LETO rats, and these changes were reversed by rosiglitazone treatment.
Figures and Tables
Fig. 1
Effects of rosiglitazone (RGZ) on hepatic triglyceride (TG) content. *p < 0.05 vs. LETO rat. †p < 0.05 vs. OLETF rat.

Fig. 2
Effects of rosiglitazone (RGZ) on liver histology of OLETF rats. Liver histology of LETO rats (A, D), untreated OLETF rats (B, E), and OLETF rats treated with rosiglitazone (C, F), stained by hematoxylin-eosin staining (× 400; A, B, C). and oil-red staining (D, E, F), respectively.

Fig. 3
Effects of rosiglitazone (RGZ) treatment on sterol regulatory binding protein-1c and fatty acid synthase expression in the liver tissue of OLETF rat. *p < 0.05 vs. LETO rat; †p < 0.05 vs. OLETF rat.
References
1. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999. 116:1413–1419.
2. Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C, Weltman M, George J. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002. 35:373–379.

3. Akyuz F, Demir K, Ozdil S, Aksoy N, Poturoglu S, Ibrisim D, Kaymakoglu S, Besisik F, Boztas G, Cakaloglu Y, Mungan Z, Cevikbas U, Okten A. The effects of rosiglitazone, metformin, and diet with exercise in nonalcoholic fatty liver disease. Dig Dis Sci. 2007. 52:2359–2367.
4. Khashab M, Chalasani N. Use of insulin sensitizers in NASH. Endocrinol Metab Clin North Am. 2007. 36:1067–1087.

5. Tahan V, Eren F, Avsar E, Yavuz D, Yuksel M, Emekli E, Imeryuz N, Celikel C, Uzun H, Haklar G, Tozun N. Rosiglitazone attenuates liver inflammation in a rat model of nonalcoholic steatohepatitis. Dig Dis Sci. 2007. 52:3465–3472.

6. Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003. 38:1008–1017.
7. Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999. 277:E1–E10.
8. Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005. 54:1331–1339.

9. Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002. 277:25226–25232.

10. Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006. 55:2277–2285.

11. Lessard SJ, Chen ZP, Watt MJ, Hashem M, Reid JJ, Febbraio MA, Kemp BE, Hawley JA. Chronic rosiglitazone treatment restores AMPKalpha2 activity in insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab. 2006. 290:E251–E257.
12. Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004. 314:580–585.

13. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001. 108:1167–1174.

14. Kakuma T, Lee Y, Higa M, Wang Z, Pan W, Shimomura I, Unger RH. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci U S A. 2000. 97:8536–8541.

15. Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002. 415:339–343.

16. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992. 41:1422–1428.

17. Man ZW, Hirashima T, Mori S, Kawano K. Decrease in triglyceride accumulation in tissues by restricted diet and improvement of diabetes in Otsuka Long-Evans Tokushima fatty rats, a non-insulin-dependent diabetes model. Metabolism. 2000. 49:108–114.

18. Seo YS, Kim JH, Jo NY, Choi KM, Baik SH, Park JJ, Kim JS, Byun KS, Bak YT, Lee CH, Kim A, Yeon JE. PPAR agonists treatment is effective in a nonalcoholic fatty liver disease animal model by modulating fatty-acid metabolic enzymes. J Gastroenterol Hepatol. 2008. 23:102–109.

19. Hockings PD, Changani KK, Saeed N, Reid DG, Birmingham J, O'Brien P, Osborne J, Toseland CN, Buckingham RE. Rapid reversal of hepatic steatosis, and reduction of muscle triglyceride, by rosiglitazone: MRI/S studies in Zucker fatty rats. Diabetes Obes Metab. 2003. 5:234–243.

20. Saadeh S, Younossi ZM. The spectrum of nonalcoholic fatty liver disease: from steatosis to nonalcoholic steatohepatitis. Cleve Clin J Med. 2000. 67:96–97. 101–104.

21. Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O'Brien PE, Dixon JB, Cameron-Smith D, Kemp BE, Steinberg GR. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005. 90:3665–3672.

22. Misra P, Chakrabarti R. The role of AMP kinase in diabetes. Indian J Med Res. 2007. 125:389–398.
24. Misra P. AMP activated protein kinase: a next generation target for total metabolic control. Expert Opin Ther Targets. 2008. 12:91–100.

25. Davies SP, Carling D, Munday MR, Hardie DG. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem. 1992. 203:615–623.
26. Yang J, Maika S, Craddock L, King JA, Liu ZM. Chronic activation of AMP-activated protein kinase-alpha1 in liver leads to decreased adiposity in mice. Biochem Biophys Res Commun. 2008. (in press).

27. Hubert A, Husson A, Chedeville A, Lavoinne A. AMP-activated protein kinase counteracted the inhibitory effect of glucose on the phosphoenolpyruvate carboxykinase gene expression in rat hepatocytes. FEBS Lett. 2000. 481:209–212.

28. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated proteinkinase in intact cells? Eur J Biochem. 1995. 229:558–565.
30. Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, Youn JH, Lee KU. Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: comparison with PPAR-gamma activation. Diabetes. 2003. 52:2331–2337.
31. Miyazaki Y, Mahankali A, Wajcberg E, Bajaj M, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2004. 89:4312–4319.

32. Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology. 2006. 147:2432–2441.
33. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006. 355:2297–2307.

34. Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, Liang TJ, Yanovski JA, Kleiner DE, Hoofnagle JH. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004. 39:188–196.

35. Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004. 36:28–34.

36. Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda). 2006. 21:48–60.

37. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005. 1:15–25.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download