Abstract
Background
Oxidative stress is important in both diabetic complications and the development and the progression of type 2 diabetes via the effects on the pancreatic β-cells. EGCG (epigallocatechin galleate), a major constituent of green tea, has been known to have beneficial effects on various diseases through the mechanisms of antioxidant and cell signaling modulation. But, very small numbers of studies were published about the direct effects of EGCG on the pancreatic β cell lines. We performed this study to see the protective effect of EGCG on pancreatic β cell line under H2O2 and the mechanisms of this phenomenon.
Methods
We used INS-1 cells and hydrogen peroxide as an oxidative stressor. Their viabilities were verified by MTT assay and FACS. The activity of glutathione peroxidase was assessed by total glutathione quantification kit. Western blot and semi-quantitative RT-PCR for the catalase, SOD (superoxide dismutase), PI3K and Akt were performed. Functional status of INS-1 cells was tested by GSIS (glucose stimulated insulin secretion).
Results
The biological effects of EGCG were different according to its concentrations. 10 µM EGCG effectively protected hydrogen peroxide induced damage in INS-1 cells. The expression and the activity of SOD, catalase and the glutathione peroxidase were significantly increased by EGCG. EGCG significantly increased PI3K and Akt activity and its effect was inhibited partially by wortmannin. GSIS was well preserved by EGCG.
Figures and Tables
Fig. 1
The cell viability assessed by MTT assay (A) and FACS (B). Cell viability against H2O2 (80 µM) induced toxicity in INS-1 cells was different on the concentration of EGCG. In the histogram, the results obtained from four independent experiments are reported as means ± S.D. (A). FACS analysis after double staining with annexin V/propidium iodide (FL1:AnnexinV, FL2:PI). Dot plots from a representative FACS experiment are shown (B).

Fig. 2
EGCG changes antioxidant enzymes expression. Total RNA was isolated from INS-1 cells incubated for 24 h without (control; C) or with hydrogen peroxide (H2O2) and in the presence of EGCG (E). (A) Expression of MnSOD, catalase, and endogenous control GAPDH was evaluated by RT-PCR. A representative experiment of three is shown. (B) MnSOD and Catalase expression evaluated by western blot analysis. A representative experiment of three is shown. (C) Densitometric analyses of western blot are reported as means ± S.D. of the three different experiments. (D) GPx activity was evaluated by Glutathione peroxidase assay kit.
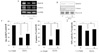
Fig. 3
EGCG modulated cell signalings related to the apoptosis. Total RNA was isolated from INS-1 cells incubated for 24 h without (control; C) or with hydrogen peroxide (H2O2) and in the presence of EGCG (E). (A) Expression of PI3K, Akt, total caspase 3, and endogenous control beta-actin was evaluated by RT-PCR. A representative experiment of three is shown. (B) Phosphorylation of PI3K and Akt and total caspase 3 were evaluated by western blot analysis. A representative experiment of three is shown. (C) Densitometric analyses of western blot are reported as means ± S.D. of the three different experiments
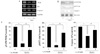
Fig. 4
The cell viability assessed by FACS after treatment of PI3K inhibitor. Cell viability against H2O2 (80 µM) induced toxicity in INS-1 cells was increased with 10 µM of EGCG (E) and partially decreased with PI3K inhibiotor (E+W). FACS analysis after double staining with annexin V/propidium iodide(FL1:AnnexinV, FL2:PI). Dot plots from a representative FACS experiment are shown (A). In the histogram, the results obtained from three independent experiments are reported as means ± S.D. (B).

References
1. Christopher JR. Type 2 Diabetes-a Matter of B cell Life and Death? Science. 2005. 307:380–384.
2. Donath MY, Ehses JA, Maedler K, Shumann DM, Ellingsgaard H, Eppler E, Reinecke M. Mechanisms of beta cell death in type 2 diabetes. Diabetes. 2005. 54:Suppl 2. S108–S113.
3. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003. 52:102–110.
4. Kahn SE. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003. 46:3–19.
5. Robertson RP, Harmon J, Tran PO, Poitout V. Beta cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004. 53:Suppl. 1. S119–S124.
6. Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia. 2002. 45:85–96.

7. Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004. 53:Suppl 1. S110–S118.

8. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003. 52:1–8.
9. Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic β-cell dysfunction. Ann N Y Acad Sci. 2004. 1011:168–176.

10. Beecher GR, Warden BA, Merken H. Analysis of tea polyphenols. Proc Soc Exp Biol Med. 1999. 220:267–270.

11. Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002. 76:560–568.

12. Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001. 7:4220–4229.
13. Liang YC, Lin-shiau SY, Chen CF, Lin JK. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (-)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J Cell Biochem. 1997. 67:55–65.

14. Sachinidis A, Seul C, Seewald S, Ahn H, Ko Y, Vetter H. Green tea compounds inhibit tyrosine phosphorylation of PDGF β-receptor and transformation of A172 human glioblastoma. FEBS Lett. 2000. 471:51–55.

15. Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (-)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005. 11:2735–2746.

16. Yang GY, Liao J, Li C, Chung J, Yurkow EJ, Ho CT, Yang CS. Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000. 21:2035–2039.
17. Peng G, Wargovich MJ, Dixon DA. Anti-proliferative effects of green tea polyphenol EGCG on Ha-Ras-induced transformation of intestinal epithelial cells. Cancer Lett. 2006. 238:260–270.

18. Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, Yang CS. Mechanism of action of (-)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005. 65:8049–8056.

19. Albrecht DS, Clubbs EA, Ferruzzi M, Bomser JA. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK- independent ERK1/2 activation. Chem Biol Interact. 2008. 171:89–95.
20. Koh SH, Kwon H, Kim KS, Kim J, Kim MH, Yu HJ, Kim M, Lee KW, Do BR, Jung HK, Yang KW, Appel SH, Kim SH. Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. Toxicology. 2004. 202:213–225.

21. Koh SH, Kim SH, Kwon H, Kim JG, Kim JH, Yang KH, Kim J, Kim SU, Yu HJ, Do BR, Kim KS, Jung HK. Phosphatidylinositol-3 kinase/Akt and GSK-3 mediated cytoprotective effect of epigallocatechin gallate on oxidative stress-injured neuronal- differentiated N18D3 cells. Neurotoxicology. 2004. 25:793–802.
22. Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (-)-epigallocatechin-3-gallate. Toxicol Appl Pharmacol. 2001. 176:110–117.

23. Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002. 277:30574–30580.
24. Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. JACC Study Group. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006. 144:554–562.

25. Tsuneki H, Ishizaka M, Terasawa M, Wu JB, Sasaoko T, Kimura I. Effect of Green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004. 4:18.
26. Sabu MC, Smitha K, Kuttan R. Anti-diabetic diabetic of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethanopharmacol. 2002. 83:109–116.
27. Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M. Epigallocatechin gallate, a constituent of Green tea represses hepatic glucose production. J Biol Chem. 2002. 277:34933–34940.

28. Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem. 2004. 52:643–648.

29. Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr. 2005. 24:376–384.

30. Doronicheva N, Yasui H, Sakurai H. Chemical structure-dependent differential effects of flavonoids on the catalase activity as evaluated by a chemiluminescent method. Biol Pharm Bull. 2007. 30:213–217.

31. Scalbert A, Morand C, Manach C, Remesy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. 2002. 56:276–282.

32. Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004. 36:838–849.

33. Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, Payrastre B. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997. 53:1649–1657.

34. Balasubramanian R, Efimova T, Eckert RL. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor -dependent involucrin gene expression in normal human keratinocytes. J Biol Chem. 2002. 277:1828–1836.
35. Agullo G, Gamet-Payrastre L, Manenti SC, Remesy C, Chap H, Payrastre B. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997. 53:1649–1657.

36. YaWen C, ChunFa H, KehSung T, RongSen Y, ChengChieh Y, ChingYao Y, ShoeiYn L, ShingHwa L. The role of phosphoinositide 3-Kinase/Akt signaling in low-dose mercury-induced mouse pancreatic β-cell dysfunction in vitro and in vivo. Diabetes. 2006. 55:1614–1624.

37. Lu M, Bi CS, Gong XG, Chen HM, Sheng XH, Deng TL, Xu KD. Anti-proliferative effects of recombinant iron superoxide dismutase on HepG2 cells via a redox-dependent PI3k/Akt pathway. Appl Microbiol Biotechnol. 2007. 76:193–201.

39. Yun SY, Kim SP, Song DK. Effects of (-)-epigallocatechin-3-gallate on pancreatic beta-cell damage in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006. 541:115–121.

40. Han MK. Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic beta-cell damage. Exp Mol Med. 2003. 35:136–139.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download