1. Kwong JC, Chua K, Charles PG. Managing severe community-acquired pneumonia due to community methicillin-resistant
Staphylococcus aureus (MRSA). Curr Infect Dis Rep. 2012; 14:330–338. PMID:
22430229.
2. Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant
Staphylococcus aureus. Clin Infect Dis. 2008; 46(Suppl 5):S378–S385. PMID:
18462093.
3. Lobo LJ, Reed KD, Wunderink RG. Expanded clinical presentation of community-acquired methicillin-resistant
Staphylococcus aureus pneumonia. Chest. 2010; 138:130–136. PMID:
20173050.
4. Jung WJ, Kang YA, Park MS, Park SC, Leem AY, Kim EY, et al. Prediction of methicillin-resistant
Staphylococcus aureus in patients with non-nosocomial pneumonia. BMC Infect Dis. 2013; 13:370. PMID:
23937553.

5. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016; 63:e61–e111. PMID:
27418577.
6. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007; 44(Suppl 2):S27–S72. PMID:
17278083.

7. Shorr AF, Myers DE, Huang DB, Nathanson BH, Emons MF, Kollef MH. A risk score for identifying methicillin-resistant
Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect Dis. 2013; 13:268. PMID:
23742753.

8. Fein AM, Niederman MS. Severe pneumonia in the elderly. Clin Geriatr Med. 1994; 10:121–143. PMID:
8168019.

9. Li W, Ding C, Yin S. Severe pneumonia in the elderly: a multivariate analysis of risk factors. Int J Clin Exp Med. 2015; 8:12463–12475. PMID:
26550157.
10. American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171:388–416. PMID:
15699079.
11. Teshome BF, Lee GC, Reveles KR, Attridge RT, Koeller J, Wang CP, et al. Application of a methicillin-resistant
Staphylococcus aureus risk score for community-onset pneumonia patients and outcomes with initial treatment. BMC Infect Dis. 2015; 15:380. PMID:
26385225.

12. Geckler RW, Gremillion DH, McAllister CK, Ellenbogen C. Microscopic and bacteriological comparison of paired sputa and transtracheal aspirates. J Clin Microbiol. 1977; 6:396–399. PMID:
334796.

13. Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998; 157:531–539. PMID:
9476869.

14. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003; 36:53–59. PMID:
12491202.
15. Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med. 1995; 333:1618–1624. PMID:
7477199.

16. Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant
Staphylococcus aureus disease in three communities. N Engl J Med. 2005; 352:1436–1444. PMID:
15814879.
17. Hageman JC, Uyeki TM, Francis JS, Jernigan DB, Wheeler JG, Bridges CB, et al. Severe community-acquired pneumonia due to
Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis. 2006; 12:894–899. PMID:
16707043.
18. Kallen AJ, Brunkard J, Moore Z, Budge P, Arnold KE, Fosheim G, et al.
Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med. 2009; 53:358–365. PMID:
18534715.
19. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, Albrecht V, Limbago B, et al. Prevalence of methicillin-resistant
Staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis. 2012; 54:1126–1133. PMID:
22438343.
20. Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant
Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005; 40:100–107. PMID:
15614698.
21. Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, et al. Association between
Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002; 359:753–759. PMID:
11888586.
22. Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, et al.
Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007; 315:1130–1133. PMID:
17234914.
23. Olsen RJ, Kobayashi SD, Ayeras AA, Ashraf M, Graves SF, Ragasa W, et al. Lack of a major role of
Staphylococcus aureus Panton-Valentine leukocidin in lower respiratory tract infection in nonhuman primates. Am J Pathol. 2010; 176:1346–1354. PMID:
20093487.
24. Peyrani P, Allen M, Wiemken TL, Haque NZ, Zervos MJ, Ford KD, et al. Severity of disease and clinical outcomes in patients with hospital-acquired pneumonia due to methicillin-resistant
Staphylococcus aureus strains not influenced by the presence of the Panton-Valentine leukocidin gene. Clin Infect Dis. 2011; 53:766–771. PMID:
21880581.
25. Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013; 188:985–995. PMID:
23855620.

26. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014; 58:330–339. PMID:
24270053.

27. Matsuda S, Ogasawara T, Sugimoto S, Kato S, Umezawa H, Yano T, et al. Prospective open-label randomized comparative, non-inferiority study of two initial antibiotic strategies for patients with nursing- and healthcare-associated pneumonia: guideline-concordant therapy versus empiric therapy. J Infect Chemother. 2016; 22:400–406. PMID:
27062334.

28. Metersky ML, Frei CR, Mortensen EM. Predictors of Pseudomonas and methicillin-resistant
Staphylococcus aureus in hospitalized patients with healthcare-associated pneumonia. Respirology. 2016; 21:157–163. PMID:
26682638.
29. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015; 373:415–427. PMID:
26172429.

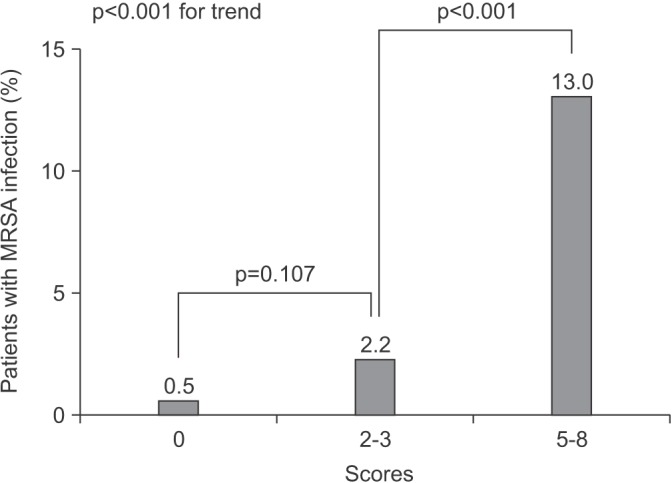
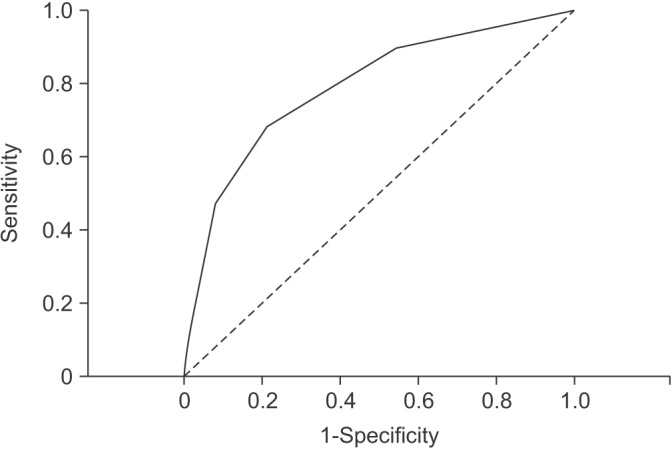
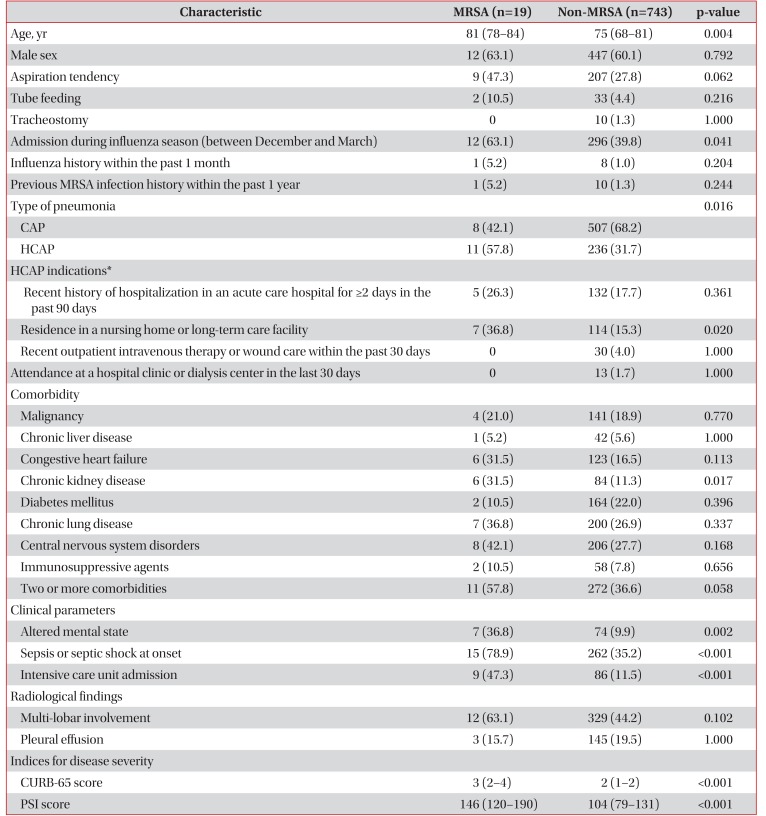
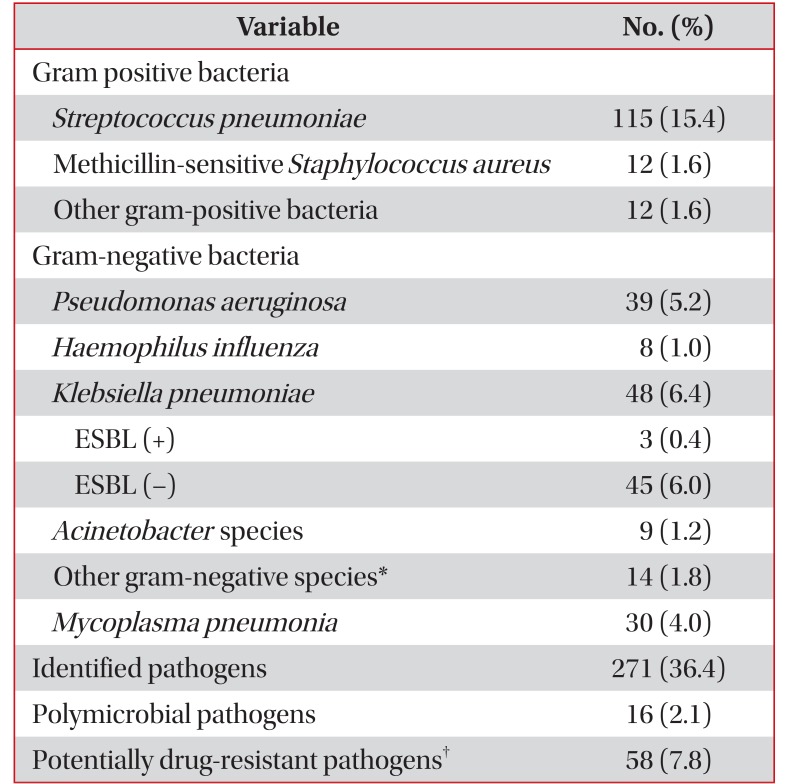




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download