Abstract
Background
Medical thoracoscopy (MT) is a minimally invasive, endoscopic procedure for exploration of the pleural cavity under conscious sedation and local anesthesia. MT has been performed at the Seoul National University Hospital since February 2014. This paper summarizes the findings and outcomes of MT cases at this hospital.
Methods
Patients who had undergone MT were enrolled in the study. MT was performed by pulmonologists, using both rigid and semi-rigid thoracoscopes. During the procedure, patients were under conscious sedation with fentanyl and midazolam. Medical records were reviewed for clinical data.
Results
From February 2014 to January 2016, 50 procedures (47 cases) were performed (diagnostic MT, 26 cases; therapeutic MT, 24 cases). The median age of patients was 66 years (59–73 years), and 38 patients (80.9%) were male. The median procedure duration from initial incision to insertion of the chest tube was 37 minutes. The median doses of fentanyl and midazolam were 50 µg and 5 mg, respectively. All procedures were performed without unexpected events. Of the 26 cases of pleural disease with an unknown cause, 19 were successfully diagnosed using MT. Additionally, diagnostic MT provided clinically useful information in the other six patients. Therapeutic MT was very effective for treatment of malignant pleural effusion or empyema. The median number of days with chest tube drainage was 6 (3 days for diagnostic MT and 8 days for therapeutic MT).
Medical thoracoscopy (MT) is a minimally invasive, endoscopic procedure for exploration of the pleural cavity under conscious sedation and local anesthesia12. MT, performed by pulmonologists, is useful as both a diagnostic and a therapeutic tool for various pleural diseases. According to currently available data, the diagnostic sensitivity of MT using rigid thoracoscopy ranges from 85% to 93%3. More recent meta-analyses have reported that the sensitivity and specificity of semi-rigid thoracoscopy are 91% and 100%, respectively4, with efficacy being as high as for surgical thoracoscopy.
As a therapeutic procedure, chemical pleurodesis performed during or after MT for recurrent and symptomatic malignant pleural effusions has a success rate of ~90%2. Furthermore, MT is useful for the treatment of pleural infection as it allows division of septations and adhesions and facilitates accurate tube placement and drainage5.
In this report, we summarize the findings and outcomes of MT performed at Seoul National University Hospital, Seoul, South Korea since February 2014.
Patients who underwent at least one MT procedure at Seoul National University Hospital, Seoul, South Korea from February 2014 to January 2016 were included in the study. MT was performed for both diagnostic and therapeutic purposes in patients with various pleural diseases. The study protocol was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB number: 1605-051-760) and was conducted in accordance with the amended Declaration of Helsinki.
All MT procedures were performed by pulmonologists under conscious sedation in the bronchoscopy room. All patients received oxygen by nasal cannula and were placed in the lateral decubitus position with the ipsilateral arm abducted and elevated over the head to maximize access to the hemithorax2. After patients were positioned, thoracic ultrasound (TUS) was used to assess the ideal entry site for the trocar. For conscious sedation, fentanyl (50 µg) and midazolam (2 mg) were administered before commencing the procedure, and an additional dose of midazolam was injected to maintain the desired level of sedation during the procedure. Local anesthesia was administered with lidocaine, and a 2–3-cm incision was made parallel to the upper margin of the rib. Blunt dissection was performed using a mosquito or Kelly clamp in the chest wall as far as the parietal pleura. Following penetration of the parietal pleura with the clamp, a trocar was introduced and the thoracoscope was placed through the trocar. A rigid thoracoscope (Olympus, Jena, Germany) or semi-rigid thoracoscope (LTF-240; Olympus, Tokyo, Japan) was used on the basis of the surgeon's preference. Upon completion of the procedure, a chest tube was inserted through the trocar, and the trocar was removed.
Procedure duration was defined as the time from skin incision to insertion of the chest tube. Tissue biopsies and/or microbiological results of samples were reviewed. Clinicians assessed the effectiveness of therapeutic MT. Major and minor complications were recorded.
Continuous variables are presented as median values (interquartile range); categorical variables, relative frequencies, and percentages. Comparison of MT for diagnosis and treatment was evaluated using the Mann-Whitney U test for continuous variables, or the chi-square test for categorical variables. A two-tailed p-value less than <0.05 indicated statistical significance. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
During a 2-year period, 47 patients underwent 50 procedures, including 26 diagnostic and 24 therapeutic MT (Figure 1). Median age of the study patients was 66 years (range, 59–73 years), and 38 patients (80.9%) were male. Almost 70% of patients had a history of smoking, and 21 had a history of malignancy (Table 1).
The median procedure time was 37 minutes, although the duration of the procedure was generally longer for patients who underwent therapeutic MT. The doses of fentanyl and midazolam that were used were different for diagnostic and therapeutic MT; fentanyl was used at a dose of 50 µg for both diagnostic and therapeutic MT, whereas midazolam was used at a dose of 5 mg for therapeutic MT and 4.5 mg for diagnostic MT (4.5 mg vs. 5 mg, p=0.048) (Table 2). All procedures were completed without unexpected events.
Nineteen of 26 cases of unexplained pleural disease were successfully diagnosed. Twelve patients were diagnosed with pleural metastases: pulmonary adenocarcinoma (five patients), non-pulmonary adenocarcinoma (one patient), squamous cell carcinoma (one patient), sarcomatoid carcinoma (one patient), small cell carcinoma (two patients), epithelioid sarcoma (one patient), and metastasis of unknown origin (one patient) (Table 2). Seven patients were diagnosed with nonmalignant diseases: subpleural abscess (one patient), tuberculous pleurisy (one patient), nontuberculous mycobacterial pleurisy (one patient), amyloidosis (one patient), graft-versus-host disease (one patient), foreign body (one patient), and immunoglobulin G4 (IgG4)-related disease (one patient) (Figure 1).
Seven patients remained undiagnosed even after MT; however, three had pleural biopsy findings that showed the presence of atypical cells, and they were clinically diagnosed with malignant pleural effusion. Finally, one patient was finally diagnosed with adenocarcinoma via video-assisted thoracoscopic surgery (VATS). Among four patients who showed nonspecific inflammation, pleural effusion was spontaneously resolved without specific treatment in three, whereas one was finally diagnosed with mesothelioma using VATS after 3 months of MT. Although a specific diagnosis was established in 19 of 26 patients, diagnostic MT provided clinically useful information in the other six. Overall diagnostic yield in the study was 96.2%.
For therapeutic purposes, 21 patients underwent 24 procedures. Ten patients with malignant pleural effusion underwent 11 procedures. One patient underwent MT twice because pleural effusion had progressed on both sides. Eleven patients with empyema underwent 13 procedures. With the exception of one procedure, therapeutic MT was generally determined to be helpful according to clinicians.
For 41 patients, the median number of days with chest tube drainage was 6 (3 days for patients who underwent diagnostic MT and 8 days for those who underwent therapeutic MT) (Table 2). The remaining patients were either discharged with the chest tube intact or died before chest tube removal. Three patients died within 30 days of MT, and underlying cancer progression was a potential cause of death.
Re-expansion pulmonary edema occurred in one patient and required drainage of 3.03 L of pleural fluid over a 3–4 hour period (Figure 2A-C). After the patient received supportive care, resolution of the edema was observed by chest radiography after 3 days (Figure 2D).
MT is a minimally invasive procedure for inspection and biopsy of the pleural space and therapeutic intervention2. MT is generally performed by pulmonologists under moderate sedation with local anesthesia in a spontaneously breathing patient; it generally takes place outside a bronchoscopy room and requires one single port of entry into the thoracic cavity. Surgical thoracoscopy, better known as VATS, is performed by a surgeon in an operating room in a patient under general anesthesia with selective intubation, and it requires at least three ports of entry into the thoracic cavity. Compared with surgical thoracoscopy, the major advantage of MT is its cost-effectiveness for patients with poor tolerance for general anesthesia6.
Patients who need therapeutic MT usually have poor lung function or performance status. MT can be performed in patients with poor lung function or those with a high anesthetic risk associated with an open procedure. Migliore et al.7 reported that MT can safely be used for patients with compromised pulmonary function. In their study, 20% of patients had a preoperative forced expiratory volume in 1 second of <1 L. Additionally, more than 60% of these patients had poor performance status (American Society of Anesthesiologists status 3 or greater)7. Therefore, MT may be a good diagnostic or therapeutic option for patients intolerant to VATS under general anesthesia.
TUS can be performed at the bedside and is very useful during MT. TUS can safely and reliably identify entry sites for trocar placement during MT, and a TUS exam adds only minutes to the duration of the procedure8. The entry site for the trocar was assessed with TUS in 78% of patients in our study, and the trocar was inserted safely without any complications. Additionally, since TUS can elucidate pleural loculations at an early stage, mechanical adhesiolysis via MT may be used as a first-line treatment without delay in patients with clear septation5. A recent study demonstrated that TUS accurately identifies intrathoracic adhesions and, in experienced hands, can guide MT access even in the complete absence of pleural effusion9.
MT is typically performed under local anesthesia, although premedication with an intravenous anxiolytic or analgesic may be accommodated10. Although sedation requirements for MT are quite low, a combination of a short-acting benzodiazepine like midazolam and an opioid, such as fentanyl, is the most commonly used strategy for moderate sedation during MT6. Previous studies have shown that the dose of midazolam used for MT is 1 to 10 mg211121314 and 50 to 100 µg for fentanyl211, which is consistent with the doses used in our study. In addition, meticulous attention to administration of local anesthesia at the entry site, especially along the periosteum, is important to achieve patient comfort and reduce pain during manipulation of the thoracoscope1210. In this retrospective, descriptive study, we investigated the outcomes of patients who underwent MT, over a 2-year period. Our findings showed that MT had a high diagnostic yield and was effective for treatment of malignant pleural effusion and empyema.
MT allows direct visual assessment of the pleura and subsequent biopsy of visually abnormal areas, which maximizes diagnostic yield3. Previous studies have reported a diagnostic yield for MT that ranges from 85% to 100%. In our study, the diagnostic yield was 96.2%, which was comparable to previous studies3415. Interestingly, one case was diagnosed as IgG4-related pleural disease in our study, and MT findings were very similar to those from a previous study16. Figure 3 shows representative features of IgG4-related pleural disease, such as severe adhesions in the pleural space (Figure 3A), thickened parietal pleura (Figure 3B), and inflamed parietal pleura with proliferating blood vessels (Figure 3C). IgG4-related disease is a recently described systemic fibro-inflammatory disease associated with elevated circulating levels of IgG417. The diagnosis of IgG4-related disease rests on the combined presence of a characteristic histopathological appearance and increased numbers of IgG4+ plasma cells18. It is known that pleural effusion is an uncommon feature of IgG4-related disease1719. However, a recent study by Murata et al.20 reported that the proportion of cases of IgG4-related pleural lesions in undiagnosed pleural effusion is estimated to be 8.3%. Therefore, IgG4-related disease in a setting of pleural effusion should be considered as a differential diagnosis of unknown cause.
Although MT has become more readily available in Europe and North America over the past two decades2, it is still an unfamiliar procedure in Korea, and only a few reports have been published there1521. Yang et al.15 reported that it was a safe method for diagnosing patients with unknown pleural effusions and had a relatively high diagnostic accuracy. However, they did not include therapeutic MT. In our study, we included patients who received therapeutic MT, which was effective in treating malignant pleural effusion and empyema.
Traditionally, diagnoses of the causes of pleural effusion or pleurodesis in patients with malignant disease are the two major indications for MT2223. According to guidelines of the British Thoracic Society, MT is not currently used in the United Kingdom either as primary or rescue therapy for pleural infection3. However, MT may be effective for managing empyema by experienced respiratory physicians23. Brutsche et al.5 reported that multiloculated empyema, as identified by ultrasonography, can safely and successfully be treated with MT. Additionally, Ravaglia et al.24 demonstrated that MT successfully treated 22 of 24 patients with multiloculated empyema (91.7%) and four of eight patients with organizing effusion (50%). In our study, more than half of the patients who underwent therapeutic MT had empyema, and one patient with empyema underwent MT twice, as this was effective for relieving symptoms such as dyspnea. Fibrinopurulent membranes of multiloculated empyema were mechanically removed with forceps to facilitate drainage of pus.
A single thoracentesis procedure using TUS rarely provides long-lasting relief. MT is more effective than conventional thoracentesis using TUS, in draining pus to provide palliation. Some patients with malignant pleural effusion have adhesions in the pleural space25, and the use of thoracoscopic adhesiolysis could manage pleural effusion more effectively. Moreover, a pleurodesis could be performed through a chest tube placed at the time of the MT procedure. Additionally, diagnostic and therapeutic MT could be performed simultaneously to treat the malignant pleural effusion. Even in patients with a known malignancy, a firm diagnosis of malignant pleural effusion is important for determining proper management because up to 50% of pleural effusions could be benign26.
In summary, MT is a useful and necessary procedure for both the diagnosis and treatment of various pleural diseases.
Figures and Tables
Figure 1
Flow diagram of patients undergoing medical thoracoscopy. *Guidewire. †Adenocarcinoma was diagnosed via video-assisted thoracoscopic surgery (VATS) in one patient; two patients were clinically diagnosed with malignant pleural effusion. ‡Mesothelioma was diagnosed via VATS in one patient, and pleural effusion was spontaneously resolved in another three patients. TB: tuberculosis; NTM: nontuberculous mycobacteria; GVHD: graft-versus-host disease.
Figure 2
Re-expansion pulmonary edema. Chest radiography immediately (A), 4 hours (B), 6 hours (C), and 3 days (D) after medical thoracoscopy.
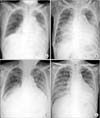
Figure 3
IgG4-related disease: findings during medical thoracoscopy. (A) Adhesions in the pleural space. (B) Thickened parietal pleura. (C) Inflamed parietal pleura with proliferating blood vessels.

Table 1
Characteristics of the study population

Table 2
Medical thoracoscopy procedure parameters

References
1. Lee P, Mathur PN, Colt HG. Advances in thoracoscopy: 100 years since Jacobaeus. Respiration. 2010; 79:177–186.
2. Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest. 2010; 138:1242–1246.
3. Rahman NM, Ali NJ, Brown G, Chapman SJ, Davies RJ, Downer NJ, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010; 65:Suppl 2. ii54–ii60.
4. Agarwal R, Aggarwal AN, Gupta D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: a meta-analysis. Chest. 2013; 144:1857–1867.
5. Brutsche MH, Tassi GF, Gyorik S, Gokcimen M, Renard C, Marchetti GP, et al. Treatment of sonographically stratified multiloculated thoracic empyema by medical thoracoscopy. Chest. 2005; 128:3303–3309.
6. Shojaee S, Lee HJ. Thoracoscopy: medical versus surgical-in the management of pleural diseases. J Thorac Dis. 2015; 7:Suppl 4. S339–S351.
7. Migliore M, Giuliano R, Aziz T, Saad RA, Sgalambro F. Four-step local anesthesia and sedation for thoracoscopic diagnosis and management of pleural diseases. Chest. 2002; 121:2032–2035.
8. Hersh CP, Feller-Kopman D, Wahidi M, Garland R, Herth F, Ernst A. Ultrasound guidance for medical thoracoscopy: a novel approach. Respiration. 2003; 70:299–301.
9. Marchetti G, Valsecchi A, Indellicati D, Arondi S, Trigiani M, Pinelli V. Ultrasound-guided medical thoracoscopy in the absence of pleural effusion. Chest. 2015; 147:1008–1012.
10. Havelock T, Teoh R, Laws D, Gleeson F. BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010; 65:Suppl 2. ii61–ii76.
11. Hallifax RJ, Corcoran JP, Ahmed A, Nagendran M, Rostom H, Hassan N, et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest. 2014; 146:1001–1006.
12. Lee P, Hsu A, Lo C, Colt HG. Prospective evaluation of flex-rigid pleuroscopy for indeterminate pleural effusion: accuracy, safety and outcome. Respirology. 2007; 12:881–886.
13. Dhooria S, Singh N, Aggarwal AN, Gupta D, Agarwal R. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care. 2014; 59:756–764.
14. Willendrup F, Bodtger U, Colella S, Rasmussen D, Clementsen PF. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions in Denmark. J Bronchology Interv Pulmonol. 2014; 21:215–219.
15. Yang JK, Lee JH, Kwon MH, Jeong JH, Lee GE, Cho HM, et al. Diagnostic accuracy and safety of medical thoracoscopy. Tuberc Respir Dis. 2007; 63:261–267.
16. Ishida A, Furuya N, Nishisaka T, Mineshita M, Miyazawa T. IgG4-related pleural disease presenting as a massive bilateral effusion. J Bronchology Interv Pulmonol. 2014; 21:237–241.
17. Ryu JH, Sekiguchi H, Yi ES. Pulmonary manifestations of immunoglobulin G4-related sclerosing disease. Eur Respir J. 2012; 39:180–186.
18. Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012; 25:1181–1192.
19. Zen Y, Inoue D, Kitao A, Onodera M, Abo H, Miyayama S, et al. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2009; 33:1886–1893.
20. Murata Y, Aoe K, Murakami T, Oishi K, Matsumoto T, Ueoka H, et al. Involvement of IgG4 in pleural effusions of unknown cause. Am J Respir Crit Care Med. 2014; 189:A5477.
21. Kim WJ, Lee HY, Lee SH, Cho SJ, Park WS, Kim JK, et al. Diagnostic accuracy of 2-mm minithoracoscopic pleural biopsy for pleural effusion. Tuberc Respir Dis. 2004; 57:138–142.
22. Lee P, Colt HG. Pleuroscopy in 2013. Clin Chest Med. 2013; 34:81–91.
23. Froudarakis ME. Medical thoracoscopy: the green shapes of grey. Chest. 2015; 147:869–871.
24. Ravaglia C, Gurioli C, Tomassetti S, Casoni GL, Romagnoli M, Gurioli C, et al. Is medical thoracoscopy efficient in the management of multiloculated and organized thoracic empyema? Respiration. 2012; 84:219–224.
25. Bielsa S, Martin-Juan J, Porcel JM, Rodriguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol. 2008; 3:1251–1256.
26. Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008; 83:235–250.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download