Abstract
Background
Although the World Health Organization (WHO) classification of lung squamous cell carcinoma (SCC) was revised in 2015, its clinical implications for lung SCC subsets remain unclear. We investigated whether the morphologic characteristics of lung SCC, including keratinization, were associated with clinical parameters and clinical outcome of patients.
Methods
A total of 81 patients who underwent curative surgical resection of diagnosed lung SCC, were enrolled in this study. Attributes such as keratinization, tumor budding, single cell invasion, and nuclear size within the tumor, as well as immunohistochemistry of Bcl-xL and pS6 expressions, were evaluated.
Results
The keratinizing and nonkeratinizing subtypes did not differ with respect to age, sex, TNM stage, and morphologic parameters such as nuclear diameter, tumor budding, and single cell invasion at the tumor edge. Most patients with the keratinizing subtype (98.0%) had a history of smoking, whereas the nonkeratinizing group had a relatively higher proportion of never-smokers relative to the keratinizing group (24.0% vs. 2.0%; p=0.008, chi-square test). Expression of pS6 (a surrogate marker of mammalian target of rapamycin complex 1 [mTORC1] signaling that regulates keratinocyte differentiation), and Bcl-xL (a key anti-apoptotic molecule that may inhibit keratinization), did not correlate significantly with the presence of keratinization. Patients with the keratinizing subtype had a significantly shorter overall survival (85.2 months vs. 135.7 months, p=0.010, log-rank test), and a multivariate analysis showed that keratinization was an independent, poor prognostic factor (hazard ratio, 2.389; 95% confidence interval, 1.090–5.233; p=0.030).
The newly published 2015 World Health Organization (WHO) classification of lung tumors reclassified squamous cell carcinomas (SCC) into keratinizing, nonkeratinizing, and basaloid subtypes1, similar to the 2005 Head and Neck WHO Classification of nasopharyngeal carcinomas. Typically, keratinization implies lung SCC, although in the absence of unequivocal keratinization, immunohistochemistry is usually required to distinguish nonkeratinizing SCC from adenocarcinoma. Similar to the head and neck cancer classification, the new lung SCC classification was upgraded to address these pathological issues2. However, the prognostic or other clinical significance of this new lung SCC subtype classification is unclear, although recent studies of head and neck cancer have revealed that compared to the non-keratinizing subtype, the keratinizing subtype is associated with a poorer prognosis34. In contrast, studies of the relationship between the keratinizing subtype and prognosis in lung SCC are rare, and one such study reported that the presence of keratinization was not a significant prognostic factor5.
According to previous literature, keratinization is accompanied by apoptosis and is ultimately associated with tumor progression in patients with esophageal SCC6. The expression of B-cell lymphoma (Bcl)-xL, an oncoprotein involved in lung SCC tumorigenesis, is known to correlate with apoptosis789; furthermore, deactivation of the tumorigenic mammalian target of rapamycin (mTOR) signaling pathway, which plays a key role in regulating cellular proliferation, survival, and angiogenesis, also affects apoptosis in lung cancers10111213. These key apoptotic factors might correlate with keratinization and thus might affect prognosis. However, potential direct correlations of keratinization with the mTOR pathway and Bcl-xL expression have not been studied in lung SCC.
In the present study, we aimed to characterize the keratinizing and nonkeratinizing subtypes of lung SCC and confirm the effects of keratinization on overall survival (OS). In addition, we aimed to investigate correlations of keratinization with mTOR pathway activation and Bcl-xL expression.
Eighty-one patients who underwent surgical treatment of lung SCC between 1993 and 2016 were randomly selected from the Severance Hospital (Seoul, Korea) lung cancer database. To obtain clinical data, we retrospectively reviewed the patients' electronic medical records. Tumor stage was re-evaluated according to the seventh edition of the American Joint Committee on Cancer TNM Staging Manual14. This study was approved by the Institutional Review Board (IRB) of Severance Hospital (No. 3-2016-0019).
All tissue slides were subjected to hematoxylin and eosin staining and evaluated for the presence of the following recently identified poor prognostic factors: tumor budding, single cell invasion, and large nuclei5. Initially, the entire tumor set was scanned at ×100 magnification and subjected to a detailed review. First, tumor budding, or the presence of small tumor nests comprising fewer than five tumor cells, were counted in 10 high-power fields (HPFs) at ×200 magnification. We defined a high grade of tumor budding as more than eight tumor budding events per 10 HPFs (Figure 1A, B). Single cell invasion was also evaluated at ×200 magnification (Figure 1C), and nuclear features were assessed at ×400 magnification. We calculated the average nuclear diameter of at least 100 tumor cells in at least three HPFs per sample. A large nucleus was defined as a diameter greater than that of four small lymphocytes (Figure 1D).
The keratinization grade was determined, and tumors were classified accordingly as the keratinizing subtype, defined as a keratinizing pattern comprising ≥5% of the entire tumor, or the nonkeratinizing subtype, defined as a keratinizing pattern comprising <5% of the tumor (Figure 1E). Subsequently, the keratinization grades were refined to nonkeratinization (<5%) and low (5%–20%), moderate (20%–50%), or severe (>50% of the entire tumor) keratinization, similar to the classification used for head and neck cancers15. The keratinization patterns included cytoplasmic keratinization (Figure 1F), keratin pearl (Figure 1G), and layered keratinization (Figure 1H). All histologic parameters were evaluated independently by a pathologist (Y.J.C.).
To evaluate mTOR complex 1 (mTORC1) and Bcl-xL expression, immunohistochemistry (IHC) staining for pS6 and Bcl-xL proteins was performed using an EnVision+ system (Dako Corp., Carpinteria, CA, USA) according to the manufacturer's instructions. Briefly, sections were deparaffinized, rehydrated, and subjected to antigen retrieval via microwave heating for 10 minutes. Sections were then immersed in a H2O2–phosphate-buffered saline solution prior to overnight incubation with primary anti-pS6 (1:400, Cell Signaling Technology, Danvers, MA, USA) or Bcl-xL antibodies (1:600, Cell Signaling Technology). Subsequently, the sections were incubated with a peroxidase-labeled polymer for 1 hour at 4℃. IHC staining was scored independently at ×200 magnification by Y.J.C. and S.H.K., who were blinded to the clinicopathological data. A semiquantitative evaluation of pS6 and Bcl-xL was performed according to the method described in a previous study16. The staining intensity was classified as 0 (negative), 1 (trace), 2 (moderate), or 3 (strong), and the frequency of positive cells was classified as 0 (<10%), 1 (10%–50%), 2 (50%–80%), or 3 (>80%). The expression score was determined as the product of the staining intensity and frequency of positive cells.
Associations between categorical variables were analyzed using the chi-square test; this test was also used to assess the linear correlation trend of the keratinization grade with IHC scoring. Disease-free survival (DFS) and OS were estimated using the Kaplan-Meier method, and associations between factors and survival outcomes (OS) were analyzed using the log-rank test. The Cox proportional hazards model was used for multivariate analyses. SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses, and significance was defined as a p-value of <0.05.
Among the 81 patients enrolled in this study, 56 (69.1%) and 25 (30.9%) were classified as having the keratinizing and nonkeratinizing subtypes of lung SCC, respectively, as confirmed by a pathologic review of tumor slides. The average age of the subjects was 62.8±8.7 years (mean±standard deviation); 77 (95.1%) were male, and the majority had stage T2 or T3 disease (84.0%). Among patients with the keratinizing subtype, 21 (37.5%), 19 (33.9%), and 16 patients (28.6%) were classified as having low, moderate, and severe keratinization, respectively. The keratinizing and nonkeratinizing groups did not differ significantly with respect to the distributions of age, sex, T-, N-, pStage, type of surgery, and adjuvant chemotherapy. However, most patients with the keratinizing subtype (98.0%) had a history of smoking, whereas never smokers (24.0%) were significantly more prevalent in the nonkeratinizing subtype group (Pearson's R=0.262, p=0.008, chi-square test). In a subgroup analysis of smokers, keratinization was more frequently observed among heavy smokers (i.e., ≥30 pack-year history of smoking) (p=0.012, chi-square test). This finding suggests that keratinization of lung SCC might be related to smoking (Table 1). Other morphologic parameters, such as nuclear diameter, tumor budding, and single cell invasion at the tumor edge, were not found to correlate with the keratinization of lung SCC.
Because mTOR signaling is known to regulate keratinocyte differentiation, we reviewed the relationship between keratinization and mTORC1 in 51 available lung SCC tissues, using pS6 expression as a surrogate marker. Among these 51 cases, five (9.8%), 12 (23.5%), and 23 (45.1%) exhibited strong, moderate, and trace expression, respectively. An additional 11 cases (21.6%) were negative for pS6. Viable tumors expressed pS6 beneath the shedding area, where keratinization initiates, regardless of keratinization subtype (Figure 2). However, the correlation between keratinization grade and pS6 expression failed to reach statistical significance (p=0.153, chi-square test).
In tumors, keratinization is generated by the epithelial layer and is subsequently shed from the tumor margin by apoptosis. Under a hypothesis that Bcl-xL, a key anti-apoptotic molecule, might inhibit keratinization, we investigated the relationship between keratinization and apoptosis in the keratinized area. Although we observed a negative correlation between Bcl-xL and tumor cell keratinization, this relationship did not reach statistical significance (Pearson's R=−0.264, p=0.062, chi-square test) (Figure 2).
We next analyzed the clinical outcomes of lung SCC according to keratinization. Although the keratinizing subtype was associated with a reduced DFS, this difference did not reach statistical significance (119.6 months vs. 122.7 months, p=0.459, log-rank test). However, the keratinizing subtype was associated with significantly shorter OS relative to the non-keratinizing subtype (85.2 months vs. 135.7 months, p=0.010, log-rank test) (Figure 3).
In a univariate analysis, old age (p=0.039) and a history of smoking (p=0.036) were associated with a significantly shorter OS. Among histologic parameters, a high tumor budding grade (p=0.037), presence of single cell invasion (p=0.006), and keratinizing subtype (p=0.013) were associated with a significantly poorer OS. To confirm keratinization as an independent prognostic factor for OS in lung SCC, we performed a multivariate analysis that included age, sex, smoking status, tumor stage, and keratinization. This analysis identified older age (>65 years; hazard ratio [HR], 2.154; 95% confidence interval [CI], 1.150–4.035; p=0.017), advanced stage (stage III; HR, 2.558; 95% CI, 1.207–5.422; p=0.014), and keratinization (HR, 2.389; 95% CI, 1.090–5.233; p=0.030) as independent poor prognostic factors (Table 2).
Keratins are epithelium-specific intermediate filament proteins that play a central role in maintaining the structural integrity of cells and are thought to be involved in cell differentiation. Keratinocytes follow a unique program of terminal differentiation and apoptotic cell death, ultimately leading to the formation of the keratin layer17. For these reasons, keratinization within tumors is considered a marker of well-differentiated SCC of the head and neck, as well as of the lungs1819.
Studies on head and neck SCC showed an association between the keratinizing subtype and a poorer OS (compared with the nonkeratinizing subtype)42021. Although the underlying mechanism remains unclear, some researchers have indicated that keratinization is associated with poor prognosis in human papillomavirus-negative head and neck SCC22. Few studies have addressed OS with respect to keratinization in lung SCC. A recent study provided a key finding regarding the effects of keratinization on OS in this cancer type. Specifically, Xiao et al.23 demonstrated that IKKα is expressed at low and high levels in keratinizing and nonkeratinizing lung SCC, respectively. As a higher level of IKKα gene expression was found to correlate with good OS in patients with lung cancer, keratinizing lung SCC might lead to a poorer OS. Furthermore, among cases of nonkeratinizing lung SCC, the differentiated subtype correlated significantly with higher IKKα expression relative to the undifferentiated subtype; in other words, keratinization of lung SCC might correlate with a poor OS, regardless of the differentiation status23.
Smoking affects both the immunologic system and keratinization process24; specifically, increased keratinization of the oral cavity is frequently observed in smokers because epithelial cells exposed to cigarette smoke defend themselves via elevated cytokeratin levels252627. The present study therefore demonstrated similar effects of smoking on keratinization in the lungs.
mTOR and Bcl-xL, which are known to associate with keratinocyte differentiation and apoptosis, respectively, were identified and analyzed in previous studies2829. mTORC1 not only plays roles in mesenchymal cell differentiation processes such as adipogenesis, osteogenesis, and myogenesis, but is also involved in epithelial cell differentiation and integrity maintenance. Although the correlation between keratinization and mTORC1 activity (per the surrogate marker pS6) did not reach statistical significance in our study, we observed some overlap of positive pS6 staining with keratinization. Areas of keratinization initiation correlated negatively with the expression of the anti-apoptotic protein Bcl-xL, consistent with previous studies that demonstrated keratinization progression at the point of apoptotic pathway activation6. Accordingly, we suggest that apoptosis is inevitable during the process of keratinization; however, additional detailed studies of the underlying mechanisms should be conducted to confirm this hypothesis.
We must note some limitations of the present study. For example, the number of study subjects was small. In addition, these subjects were limited to patients with surgically resectable lung SCC. A large cohort study that includes patients with unresectable lung SCC, as well as molecular profiling, will be needed to identify the detailed underlying mechanisms. Nevertheless, this study demonstrated that keratinization is a significantly poor prognostic factor in lung SCC, thus warranting large-scale studies to confirm this finding.
Herein, we demonstrated the association of keratinization of lung SCC with a poor clinical outcome, in comparison with the nonkeratinization subtype. This outcome might result from the association of keratinization with smoking and the apoptotic features usually observed at the keratinizing site.
Figures and Tables
Figure 1
Histologic parameters and patterns of keratinization applied in this study. Tumor budding is observed along tumor edge (A); this is defined as the presence of structures comprising fewer than five tumor cells (arrowheads) at higher magnification (B). Single cell invasion (C) and large nuclei (cancer cell nucleus >4 times than that of a small lymphocyte) (D), nonkeratinization (entire tumor area, <5% keratinization) (E), cytoplasmic keratinization (F), keratin pearl (G), and layered keratinization (H) (H&E stain; A, ×40; B–D, ×400; E–H, ×200).
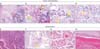
Figure 2
Relationship of keratinization (A–D, H&E stain, ×200) with the expression of pS6 ribosomal protein (Ser235/236; E–H, ×200) and Bcl-xL (I–L, ×200). Images represent nonkeratinization (A, E, I), cytoplasmic keratinization (B, F, J), keratin pearl (C, G, K), and layered keratinization (D, H, L). Distribution of pS6 (M) and Bcl-xL (N) expression in lung squamous cell carcinoma.
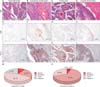
Figure 3
Disease-free survival (A) and overall survival (B) of patients with lung squamous cell carcinoma according to the keratinization status (p-values were obtained using the log-rank test).

Table 1
Demographics and pathologic characteristics of patients with and without keratinization

Table 2
Univariate and multivariate analyses of overall survival

Acknowledgments
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (grant No. NRF-2015R1C1A1A02037675) given to EY Kim.
References
1. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, et al. The 2015 World Health Organization Classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015; 10:1243–1260.
2. Cardesa A, Nadal A. Carcinoma of the head and neck in the HPV era. Acta Dermatovenerol Alp Pannonica Adriat. 2011; 20:161–173.
3. Yoshikawa H, Ehrhart EJ, Charles JB, Custis JT, LaRue SM. Assessment of predictive molecular variables in feline oral squamous cell carcinoma treated with stereotactic radiation therapy. Vet Comp Oncol. 2016; 14:39–57.
4. Cooper T, Biron VL, Adam B, Klimowicz AC, Puttagunta L, Seikaly H. Association of keratinization with 5-year disease-specific survival in oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015; 141:250–256.
5. Kadota K, Nitadori J, Woo KM, Sima CS, Finley DJ, Rusch VW, et al. Comprehensive pathological analyses in lung squamous cell carcinoma: single cell invasion, nuclear diameter, and tumor budding are independent prognostic factors for worse outcomes. J Thorac Oncol. 2014; 9:1126–1139.
6. Ohbu M, Saegusa M, Okayasu I. Apoptosis and cellular proliferation in oesophageal squamous cell carcinomas: differences between keratinizing and nonkeratinizing types. Virchows Arch. 1995; 427:271–276.
7. Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997; 3:614–620.
8. Lu QL, Abel P, Foster CS, Lalani EN. bcl-2: role in epithelial differentiation and oncogenesis. Hum Pathol. 1996; 27:102–110.
9. Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S, Navab R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One. 2014; 9:e103698.
10. Wiedmann MW, Caca K. Molecularly targeted therapy for gastrointestinal cancer. Curr Cancer Drug Targets. 2005; 5:171–193.
11. Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002; 8:128–135.
12. Zhu Q, Liang X, Dai J, Guan X. Prostaglandin transporter, SLCO2A1, mediates the invasion and apoptosis of lung cancer cells via PI3K/AKT/mTOR pathway. Int J Clin Exp Pathol. 2015; 8:9175–9181.
13. Jeong EH, Choi HS, Lee TG, Kim HR, Kim CH. Dual inhibition of PI3K/Akt/mTOR pathway and role of autophagy in non-small cell lung cancer cells. Tuberc Respir Dis (Seoul). 2012; 72:343–351.
14. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.
15. Wenig BM, Cohen JM. General principles of management of head and neck cancer. In : Harrison LB, Sessions RB, Hong WK, editors. Head and neck cancer: a multidisciplinary approach. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;2009. p. 3–50.
16. Kim EY, Kim A, Kim SK, Kim HJ, Chang J, Ahn CM, et al. KRAS oncogene substitutions in Korean NSCLC patients: clinical implication and relationship with pAKT and RalGTPases expression. Lung Cancer. 2014; 85:299–305.
17. Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochim Biophys Acta. 2013; 1833:3471–3480.
18. Yoshida N, Egami H, Yamashita J, Takai E, Tamori Y, Fujino N, et al. Immunohistochemical expression of SKALP/elafin in squamous cell carcinoma of human lung. Oncol Rep. 2002; 9:495–501.
19. Das DK, Chakraborty C, Sawaimoon S, Maiti AK, Chatterjee S. Automated identification of keratinization and keratin pearl area from in situ oral histological images. Tissue Cell. 2015; 47:349–358.
20. Crissman JD, Pajak TF, Zarbo RJ, Marcial VA, Al-Sarraf M. Improved response and survival to combined cisplatin and radiation in non-keratinizing squamous cell carcinomas of the head and neck. An RTOG study of 114 advanced stage tumors. Cancer. 1987; 59:1391–1397.
21. Cai C, Chernock RD, Pittman ME, El-Mofty SK, Thorstad WL, Lewis JS Jr. Keratinizing-type squamous cell carcinoma of the oropharynx: p16 overexpression is associated with positive high-risk HPV status and improved survival. Am J Surg Pathol. 2014; 38:809–815.
22. Slebos RJ, Jehmlich N, Brown B, Yin Z, Chung CH, Yarbrough WG, et al. Proteomic analysis of oropharyngeal carcinomas reveals novel HPV-associated biological pathways. Int J Cancer. 2013; 132:568–579.
23. Xiao D, Jia J, Shi Y, Fu C, Chen L, Jiang Y, et al. Opposed expression of IKKalpha: loss in keratinizing carcinomas and gain in non-keratinizing carcinomas. Oncotarget. 2015; 6:25499–25505.
24. Michcik A, Cichorek M, Daca A, Chomik P, Wojcik S, Zawrocki A, et al. Tobacco smoking alters the number of oral epithelial cells with apoptotic features. Folia Histochem Cytobiol. 2014; 52:60–68.
25. Ahmed HG, Ebnoof SO, Hussein MO, Gbreel AY. Oral epithelial atypical changes in apparently healthy oral mucosa exposed to smoking, alcohol, peppers and hot meals, using the AgNOR and Papanicolaou staining techniques. Diagn Cytopathol. 2010; 38:489–495.
26. Orellana-Bustos AI, Espinoza-Santander IL, Franco-Martinez ME, Lobos-James-Freyre N, Ortega-Pinto AV. Evaluation of keratinization and AgNORs count in exfoliative cytology of normal oral mucosa from smokers and non-smokers. Med Oral. 2004; 9:197–203.
27. Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000; 11:383–408.
28. Xiang X, Zhao J, Xu G, Li Y, Zhang W. mTOR and the differentiation of mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai). 2011; 43:501–510.
29. Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998; 16:395–419.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download