Abstract
Background
Recently, increased levels of high-mobility group box 1 protein (HMGB1) have been identified in various inflammatory conditions and infections. However, no studies have evaluated the HMGB1 level in nontuberculous mycobacterial (NTM) lung disease, and compared it to mycobacterial lung disease.
Methods
A total of 60 patients newly diagnosed with NTM lung disease, 44 culture-positive pulmonary tuberculosis (TB) patients, and 34 healthy controls, were included in this study. The serum HMGB1 concentrations were quantified using HMGB1 enzyme-linked immunosorbent assay kits.
Results
Serum HMGB1 level in patients with pulmonary TB or NTM lung disease, was significantly lower than that of the healthy controls. In addition, the serum HMGB1 level in TB patients was significantly lower than patients with NTM lung disease. However, the levels in NTM patient subgroups did not differ according to the causative species, disease progression, and disease phenotype.
All members of the genus Mycobacterium other than M. tuberculosis (MTB) complex and M. leprae are considered nontuberculous mycobacteria (NTM), and are frequently isolated from environmental sources, including surface or tap water and soil12. Currently, more than 170 mycobacterial species/subspecies are listed in the Genus Mycobacterium database (http://www.bacterio.cict.fr/m/mycobacterium.html), and the number of newly identified NTM is increasing. The number of patients with pulmonary disease caused by NTM has been increasing worldwide, including South Korea34.
High-mobility group box 1 protein (HMGB1) was originally reported as a DNA-binding protein involved in DNA recombination, repair, and gene transcription5. HMGB1 is released into the extracellular milieu from necrotic cells and activated macrophages with accompanying organ failure. HMGB1 then migrates to tissue sites and induces various inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, IL-6, IL-8, macrophage inflammatory protein (MIP)-1α, and MIP-1β, thereby promoting chronic inflammation67. Several studies have described the roles of HMGB1 in pathological states, including atherosclerosis, arthritis, acute lung injury, and bacterial pneumonia8910. A few papers have reported that the serum level of HMGB1 was increased in patients with tuberculosis (TB)1112. However, only limited information is currently available about the role of HMGB1 in TB infection. Moreover, HMGB1 level has not been evaluated in NTM lung disease.
Therefore, the aim of this study was to compare serum HMGB1 level in patients with mycobacterial lung disease including pulmonary TB and NTM lung disease and to evaluate the association between serum HMGB1 level in patients with NTM lung disease at diagnosis time and etiological agent or disease progression.
The data in the present study are part of an ongoing prospective observational cohort study investigating NTM lung disease (ClinicalTrials.gov Identifier: NCT00970801). The study protocol was approved through the Institutional Review Board of Samsung Medical Center (IRB approval 2008-09-016), and written informed consent was obtained from all participants. A total of 60 patients that were newly diagnosed with NTM lung disease from January 2007 to October 2011 at Samsung Medical Center were enrolled. A diagnosis of NTM lung disease was made in patients who fulfilled the clinical, radiographic, and microbiological diagnostic criteria published by the American Thoracic Society1. NTM species were identified by polymerase chain reaction (PCR)–restriction fragment length polymorphism analysis or PCR–reverse blot hybridization assay (REBA Myco-ID; YD Diagnostics, Yongin, Korea) based on the rpoB gene at time of diagnosis131415.
Thirty-four healthy controls (HC) with no previous history of pulmonary disease and 44 patients with pulmonary TB were also tested. Pulmonary TB was diagnosed by clinical findings, chest radiographs, sputum analysis by standard microscopy for acid fast bacilli (AFB), culture for MTB using standard methods (solid media and on liquid media), and PCR using a Cobas TaqMan MTB test (Roche Diagnostics, Basel, Switzerland) or Xpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA)1617. Control samples were obtained from subjects who visited the health promotion center for regular health checkups without any clinical symptoms or signs of illness due to mycobacterial infection.
Serum samples were carefully collected from the HC and from patients with NTM lung disease and pulmonary TB before treatment and stored at −80℃ until testing. The HMGB1 concentrations in the sera were quantified using an HMGB1 ELISA kit (IBL International, Hamburg, Germany) according to the manufacturer's instructions. Briefly, 100 µL sample diluent was added to each well and then 10 µL standard, sample or control was added to the well. The plate was incubated for 24 hours at 37°C. After washing, 100 µL/well of anti-HMGB1 antibody conjugated to peroxidase was added and the plate was incubated at room temperature for 2 hours. After washing, substrate was added to each well. The enzyme reaction was incubated for 30 minutes at room temperature. The chromogenic substrate reaction was stopped by the addition of stop solution. Absorbance was determined using an automated plate reader at an excitation wavelength of 450 nm.
The data are presented as numbers (percentages) for categorical variables and medians (interquartile range [IQR]) for continuous variables. Because the data were nonparametric, Tukey's box and whisker plots were used to show centrality and dispersion. Statistical analyses were performed using the Kruskal-Wallis test or the Mann-Whitney U test with post-hoc paired comparisons using the Bonferroni method. All statistical analyses were performed using PASW version 18.0 (SPSS Inc., Chicago, IL, USA). p-values less than 0.05 were considered statistically significant.
The baseline characteristics of patients with NTM lung disease are summarized in Table 1. The median age was 57 years (IQR, 48–66 years), and 36 (60%) were female. The median body mass index was 20.2 kg/m2 (IQR, 18.9–21.7 kg/m2). No patients had immunodeficiency, and all subjects were negative for human immunodeficiency virus antibodies. The etiological agents included M. avium in 20 patients (33%), M. intracellulare in 20 patients (33%), M. abscessus in 10 patients (17%), and M. massiliense in 10 patients (17%). Forty-five patients (75%) had nodular bronchiectatic disease, 14 (23%) had fibrocavitary disease, and one (2%) had an unclassifiable form of disease.
Serum levels of HMGB1 were different among patients with NTM lung disease (median, 2.17 ng/mL; IQR, 1.34–3.71 ng/mL), TB (median, 0.76 ng/mL; IQR, 0.34–1.68 ng/mL), and the HC (median, 3.21 ng/mL; IQR, 2.21–5.15 ng/mL; p<0.001) (Figure 1A). Serum HMGB1 level in NTM lung disease and TB patients was significantly lower than that in the HC (p<0.01 and p<0.001, respectively). In addition, serum HMGB1 level in TB patients was significantly lower than that in NTM lung disease patients (p<0.001).
Subgroup analysis of 60 newly diagnosed NTM patients was performed to evaluate the association between serum HMGB1 level and etiological agents. The serum levels of HMGB1 were not different according to etiology (p=0.585) (Figure 1B) in patients with NTM lung disease.
NTM lung disease may progress slowly. Furthermore, patients may not require treatment or may require combination antibiotic therapy. After this information was discussed with the patients, an observation period of at least 6 to 12 months without antibiotic treatment usually was implemented. When the disease was clearly recognized as progressive, the patients received combination antibiotic therapy. In patients with serious symptoms and advanced or progressive radiographic abnormalities, antibiotic therapy was initiated immediately. Disease progression was determined by observing patients with NTM lung disease until combination antibiotic therapy was initiated. Among the 60 patients, 41 patients were received combination antibiotic therapy following a diagnosis of progressive NTM lung disease. The median time interval between diagnosis of NTM lung disease and start of antibiotic therapy in progression group was 90 days (IQR, 27.5–383.5 days). Nineteen patients underwent long-term follow-up without treatment for stable NTM lung disease. The serum levels of HMGB1 were not different according to disease progression (p=0.489) (Figure 1C) in patients with NTM lung disease.
There was no difference according to types of NTM disease (fibrocavitary form [median, 1.93 ng/mL; IQR, 1.19–3.79 ng/mL] vs. nodular bronchiectatic form [median, 2.27 ng/mL; IQR, 1.39–3.77 ng/mL], p=0.748), and AFB smear status (AFB smear positive [median, 2.15 ng/mL; IQR, 1.58–3.67 ng/mL] vs. AFB smear negative [median, 2.27 ng/mL; IQR, 0.90–3.71 ng/mL], p=0.495). In addition, there was no correlation between C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) and HMGB1 in both NTM (p=0.066 and p=0.277, respectively) and TB patients (p=0.743 and p=0.556, respectively).
Because HMGB1 is released by both living and dead cells in various tissues, the detection of HMGB1 might or might not reflect disease status; as such, it is difficult to use as a unique diagnostic marker. However, HMGB1 has been suggested to be common to diverse diseases and might serve as a potential marker for understanding disease processes. As different species of mycobacteria are known to cause necrotic cell death in vitro , the release of HMGB1 from cultured cell supernatants could be passive in nature18. HMGB1 released from lung macrophages could be an important mediator of the inflammatory response and might be involved in the secretion of proinflammatory cytokines and the release of nitric oxide (NO) during pulmonary TB19.
In previous reports, HMGB1 level was significantly increased in patients with TB compared with HC (TB patients vs. HC, 47.5±26.1 ng/mL vs. 13.8±7.3 ng/mL; roughly 2.2 ng/mL vs. 1.3 ng/mL [mean values not shown], respectively)1112. There was a large difference between the HMGB1 levels between the two studies. In many papers, the serum/plasma HMGB1 levels were reported to be 1.12 to 3.9 ng/mL greater than the mean or median, respectively, in HC202122. In fact, HMGB1 can be released when preparing the serum due to hemolysis and activated cells, so it is important to immediately collect the serum. The median HMGB1 levels in the HC in our study were similar to those in the published literature. In the present study, unlike in previous reports examining TB and other pulmonary infections, the serum HMGB1 levels in patients with TB and NTM lung disease were significantly lower than those in the HC. HMGB1, a late-phase mediator of inflammation, is induced by early proinflammatory cytokines and triggers the pathological and immunosuppressive effects that accompany the subsequent release of cytokines such as TNF-α, IL-1α, IL-1β, IL-6, and IL-8 during late infection23. In our previous studies, the serum levels of T helper type 1-related cytokines were significantly decreased in patients with NTM lung disease compared with the HC2425. Therefore, the down-regulated levels of HMGB1 in patients with NTM lung disease might be influenced by the decreased levels of proinflammatory cytokines. HMGB1 can be reduced or oxidized; reduced HMGB1 is a cytokine-stimulating factor that induces TNF-α release and nuclear factor κB activation, while oxidized HMGB1 induces immunosuppressive effects26. During early infection, the activated macrophages produce NO, which creates a highly oxidizing environment. Apoptotic macrophages liberate oxidized HMGB1 and temporally suppress excessive inflammation and decrease protective immunity. During late disease, the oxidative environment decreases as a result of weaker macrophage activation, lower NO production, and reduced macrophage apoptosis, leading to reduced HMGB1 production27.
The serum levels of HMGB1 were not different according to disease progression in patients with NTM lung disease. In addition, there was no difference according to severities of disease such as types of NTM disease and AFB smear status and no correlation between CRP or ESR and HMGB1 in both NTM. Depending on these results, at least HMGB1 might not be a predictor for disease progression, disease type, or the status of inflammation in NTM lung disease.
In countries with classically high TB prevalence rates, like South Korea, those patients with AFB-positive sputum on direct microscopic examination have generally been presumed to have pulmonary TB and were treated empirically. Therefore, an early differentiation between pulmonary TB and NTM lung disease in patients with AFB smear-positive specimens is very important in South Korea2829. Biomarker discovery and validation studies are needed to define combinations of markers that might eventually assist case identification and accelerate access to treatment30. The identification of a pattern of differentially expressed biomarker could help to differentiate TB infection from the NTM infection or non-infected state.
There is some inconsistency in the serum HMGB1 levels among various infectious and inflammatory diseases. For example, the elevated levels of HMGB1 are present in synovial fluid from rheumatoid arthritis patients1031. However, in sera from most rheumatoid arthritis patients, they could not detect HMGB1, whereas HMGB1 was detected in some healthy subjects32. Serum HMGB1 level in rheumatoid arthritis patients was significantly lower than that of the HCs32. In another study, serum HMGB1 level of patients infected with Legionella pneumonia was not different to that in HCs33. This finding disagrees with the results of previous studies in which serum HMGB1 was higher in community-acquired infections and pneumonia than in healthy subjects343536.
Our study had several limitations. First, this study included a relatively small number of patients. Second, HMGB1 protein could correlate with the degree of inflammatory markers, such as serum ESR and CRP. However, this information was not available in substantial proportion of patients with pulmonary TB. Third, patients with the early stage of NTM lung disease, especially the nodular bronchiectatic form, could have normal chest X-ray findings. Therefore, the possibility of early stage of NTM lung disease in control subjects could not be completely ruled out in our study.
To the best of our knowledge, this study is the first to determine that serum HMGB1 is lower in patients with NTM lung disease compared to HC. Furthermore, the low level of HMGB1 in pulmonary TB patients suggests that the magnitude of serum HMGB1 has the potential to differentiate between different etiologies of mycobacterial lung disease. Further large, prospective studies are needed to understand the implications of biosignatures for development of future differential biomarkers for mycobacterial lung disease. Conflicts of Interest No potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C2778 and HR14C0006).
References
1. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416. PMID: 17277290.

2. Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis. 2016; 45:123–134. PMID: 26976549.

3. Kwon YS, Koh WJ. Diagnosis of pulmonary tuberculosis and nontuberculous mycobacterial lung disease in Korea. Tuberc Respir Dis. 2014; 77:1–5.

4. Ryu YJ, Koh WJ, Daley CL. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians' perspectives. Tuberc Respir Dis. 2016; 79:74–84.

5. Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999; 19:5237–5246. PMID: 10409715.

6. O'Connor KA, Hansen MK, Rachal Pugh C, Deak MM, Biedenkapp JC, Milligan ED, et al. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine. 2003; 24:254–265. PMID: 14609567.
7. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002; 418:191–195. PMID: 12110890.

8. Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000; 165:2950–2954. PMID: 10975801.
9. Kosai K, Seki M, Yanagihara K, Nakamura S, Kurihara S, Izumikawa K, et al. Elevated levels of high mobility group box chromosomal protein-1 (HMGB-1) in sera from patients with severe bacterial pneumonia coinfected with influenza virus. Scand J Infect Dis. 2008; 40:338–342. PMID: 17918013.

10. Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003; 48:971–981. PMID: 12687539.

11. Magrys A, Paluch-Oles J, Koziol-Montewka M, Zaborowski T, Milanowski J, Maciejewska B. Evaluation of high-mobility group box 1 protein concentration in serum of patients with M. tuberculosis infection. Immunol Invest. 2013; 42:49–60. PMID: 23231044.
12. Zeng JC, Xiang WY, Lin DZ, Zhang JA, Liu GB, Kong B, et al. Elevated HMGB1-related interleukin-6 is associated with dynamic responses of monocytes in patients with active pulmonary tuberculosis. Int J Clin Exp Pathol. 2015; 8:1341–1353. PMID: 25973018.
13. Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011; 183:405–410. PMID: 20833823.
14. Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015; 191:96–103. PMID: 25393520.
15. Wang HY, Bang H, Kim S, Koh WJ, Lee H. Identification of Mycobacterium species in direct respiratory specimens using reverse blot hybridisation assay. Int J Tuberc Lung Dis. 2014; 18:1114–1120. PMID: 25189562.
16. Ryu YJ. Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberc Respir Dis. 2015; 78:64–71.

17. Leylabadlo HE, Kafil HS, Yousefi M, Aghazadeh M, Asgharzadeh M. Pulmonary tuberculosis diagnosis: where we are? Tuberc Respir Dis. 2016; 79:134–142.

18. Danelishvili L, McGarvey J, Li YJ, Bermudez LE. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol. 2003; 5:649–660. PMID: 12925134.
19. Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006; 208:261–269. PMID: 16362982.

20. Naumnik W, Nilklinska W, Ossolinska M, Chyczewska E. Serum levels of HMGB1, survivin, and VEGF in patients with advanced non-small cell lung cancer during chemotherapy. Folia Histochem Cytobiol. 2009; 47:703–709. PMID: 20430742.

21. Petrof G, Abdul-Wahab A, Proudfoot L, Pramanik R, Mellerio JE, McGrath JA. Serum levels of high mobility group box 1 correlate with disease severity in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2013; 22:433–435. PMID: 23711070.

22. Wang C, Gou SJ, Chang DY, Yu F, Zhao MH, Chen M. Association of circulating level of high mobility group box 1 with disease activity in antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Care Res (Hoboken). 2013; 65:1828–1834. PMID: 24591411.

23. Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001; 164(10 Pt 1):1768–1773. PMID: 11734424.

24. Kim SY, Koh WJ, Kim YH, Jeong BH, Park HY, Jeon K, et al. Importance of reciprocal balance of T cell immunity in Mycobacterium abscessus complex lung disease. PLoS One. 2014; 9:e109941. PMID: 25295870.
25. Kim SY, Koh WJ, Park HY, Jeon K, Kwon OJ, Cho SN, et al. Changes in serum immunomolecules during antibiotic therapy for Mycobacterium avium complex lung disease. Clin Exp Immunol. 2014; 176:93–101. PMID: 24354934.
26. Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013; 93:865–873. PMID: 23446148.

27. Hernandez-Pando R, Barrios-Payan J, Mata-Espinosa D, Marquina-Castillo B, Hernandez-Ramirez D, Bottasso OA, et al. The role of high mobility group box 1 protein (HMGB1) in the immunopathology of experimental pulmonary tuberculosis. PLoS One. 2015; 10:e0133200. PMID: 26201072.

28. Koh WJ, Yu CM, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Pulmonary TB and NTM lung disease: comparison of characteristics in patients with AFB smear-positive sputum. Int J Tuberc Lung Dis. 2006; 10:1001–1007. PMID: 16964791.
29. Kim YK, Hahn S, Uh Y, Im DJ, Lim YL, Choi HK, et al. Comparable characteristics of tuberculous and non-tuberculous mycobacterial cavitary lung diseases. Int J Tuberc Lung Dis. 2014; 18:725–729. PMID: 24903945.

30. Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011; 11:343–354. PMID: 21475309.

31. Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, Wang H, et al. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002; 46:2598–2603. PMID: 12384917.
32. Urbonaviciute V, Furnrohr BG, Weber C, Haslbeck M, Wilhelm S, Herrmann M, et al. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol. 2007; 81:67–74. PMID: 17060363.

33. Higa F, Furugen M, Koide M, Karimata Y, Nabeya D, Iha Y, et al. Clinical evaluation of high mobility group box 1 protein in Legionella pneumophila pneumonia. J Infect Chemother. 2014; 20:289–292. PMID: 24679738.
34. van Zoelen MA, Laterre PF, van Veen SQ, van Till JW, Wittebole X, Bresser P, et al. Systemic and local high mobility group box 1 concentrations during severe infection. Crit Care Med. 2007; 35:2799–2804. PMID: 17901841.

35. Gaini S, Koldkjaer OG, Moller HJ, Pedersen C, Pedersen SS. A comparison of high-mobility group-box 1 protein, lipopolysaccharide-binding protein and procalcitonin in severe community-acquired infections and bacteraemia: a prospective study. Crit Care. 2007; 11:R76. PMID: 17625012.

36. Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, et al. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007; 35:1061–1067. PMID: 17334246.

Figure 1
Levels of serum high mobility group box chromosomal protein 1 (HMGB1). (A) Amount of HMGB1 in the sera of healthy controls (HC; n=34), patients with nontuberculous mycobacteria (NTM) lung disease (n=60), and tuberculosis (TB) patients (n=44). (B) HMGB1 levels according to etiology (Mycobacterium avium [n=20], M. intracellulare [n=20], M. abscessus [n=10], and M. massiliense [n=10]). (C) HMGB1 levels according to disease progression (stable [n=19] vs. progressive [n=41]). The data are presented as Tukey's box-and-whisker plots. **p<0.01, ***p<0.001 for the results of comparisons between patients and controls.
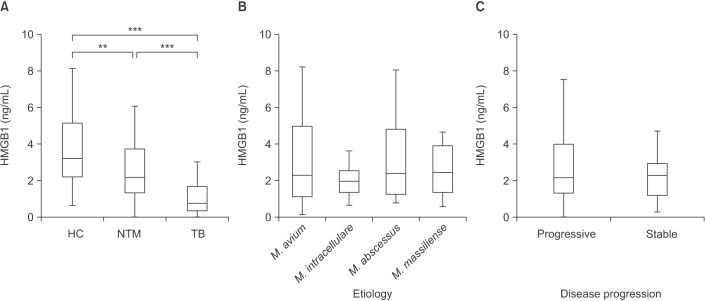
Table 1
Baseline characteristics of the 60 study patients with NTM lung disease
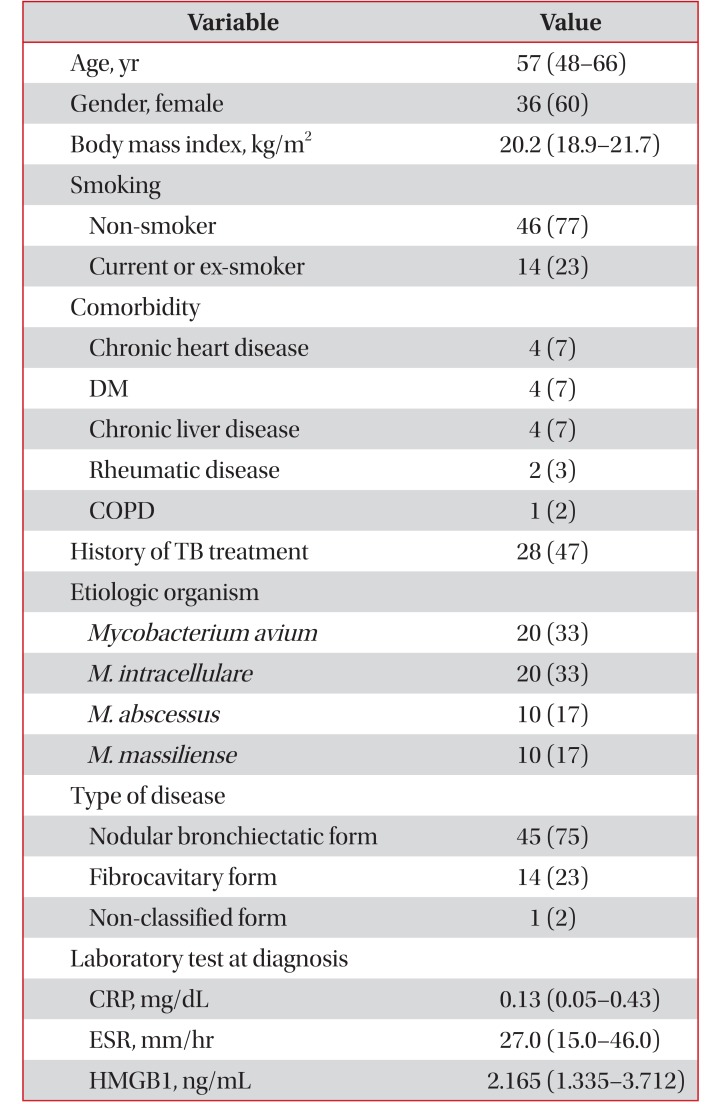
Values are presented as median (IQR) or number (%).
NTM: nontuberculous mycobacteria; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; TB: tuberculosis; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; HMGB1: high mobility group box chromosomal protein 1; IQR: interquartile range.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download